Plant physiology is the branch of botany that deals with the study of processes and functions associated with the life of plants.
The term ‘physiology’ was first used by Jean Fernel where the meaning was ‘natural knowledge’. The father of plant physiology is Stephen Hales.
Interesting Science Videos
What is Diffusion?
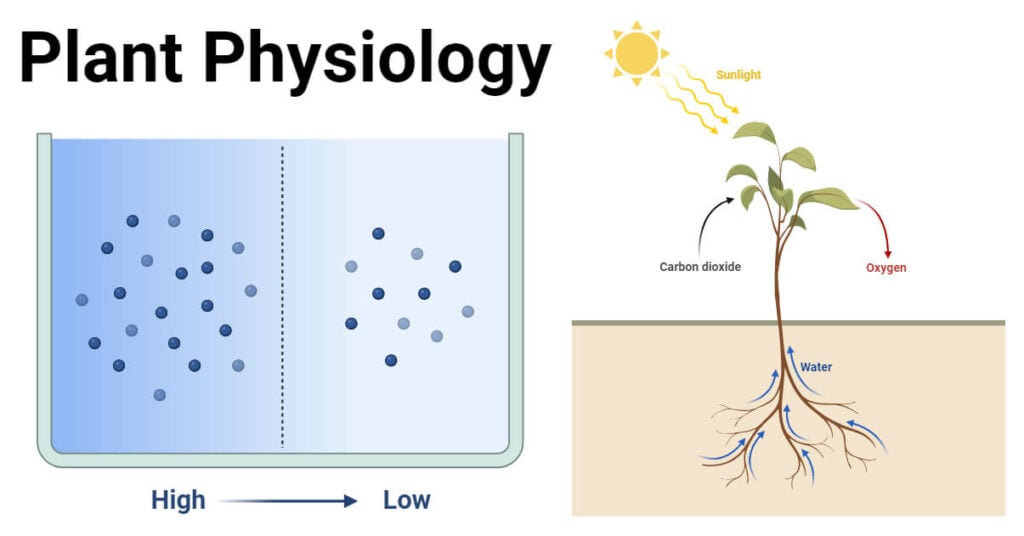
Diffusion involves random and spontaneous movement of molecules of a substance from an area of higher concentration to an area of lower concentration through a permeable membrane or a region without a barrier.
Diffusion Pressure
The pressure exerted by the diffusing molecules when two molecules of a substance move from a region of its higher concentration to a region of its lower concentration is termed as diffusion pressure. The molecules of a substance always move from a region of higher diffusion pressure to a region of lower diffusion pressure. The process of diffusion continues till the diffusion gradient exists or until the concentration in both regions becomes equalized.
Any pure substance or solvent will have the highest diffusion pressure, however, when a solute is added to the solvent, the diffusion pressure of the resultant solution decreases. The amount by which the diffusion pressure of a solution is lower than that of its pure solvent at the same temperature and pressure is called diffusion process deficit (DPD). DPD is also referred to as suction pressure (SP).
Molecules of a substance always move from a region of low DPD or SP to a region of high DPD or SP.
Independent diffusion
In a diffusion system, more than one substance diffuses through the same medium at the same time. The diffusion of one substance is not affected by the diffusion of the other substances. These substances diffuse depending on their partial pressure. This phenomenon is called independent diffusion.
Independent diffusion is important in plant life. For instance, the exchange of Carbon dioxide, Oxygen, and water vapor occurs through the stomata of the leaf through independent diffusion.
Example of Diffusion
The experiment of diffusion using Copper Sulphate in water
Requirements:
Apparatus- Beaker, Forcep
Chemical- Water, Crystal of copper sulfate
Procedure:
In this experiment, a beaker is filled with water. A small crystal of copper sulfate is placed at the bottom of it with the help of forceps and left undisturbed for an hour.
Observation:
After an hour, the color of water in the beaker turns blue. The crystal disappears in water.
Explanation and conclusion:
The concentration of copper sulfate molecules is higher in the crystal compared to water. Hence, the copper sulfate diffuses from its higher concentration to a lower concentration in water either in the form of Cu++ and So4– ions or entire molecule of CuSo4. water in the beaker gradually turns to a blue color. It results in a change in the color of water to blue.
Factors affecting diffusion
1. Temperature
The rate of diffusion of a substance increases with the increase in the temperature of the diffusing substance. This is because the increase in temperature increases the kinetic energy of the diffusing molecules.
2. Density of diffusing substance
The rate of diffusion of a substance is inversely proportional to the square root of density of the diffusing substance i.e. r ∝ 1/√d
Where,
r = rate of diffusion
d = density of different substances.
This law is Graham’s law of diffusion.
3. Density of diffusion medium
The rate of diffusion of a substance is higher in a medium of lower density compared to that of higher density. Hence, gas molecules diffuse faster in a vacuum than in air.
4. Area of diffusion
The rate of diffusion increases with an increase in the area of diffusion.
5. Diffusion Pressure gradient
The rate of diffusion increases with the increase in the diffusion pressure gradient.
Importances of Diffusion
- The exchange of oxygen and carbon dioxide through the open stomata during photosynthesis and respiration occurs by diffusion.
- Water vapor is lost from the internal parts of the plant body during transpiration by the process of diffusion.
- Diffusion is an effective means of transporting substances over short distances such as within the cell or from one cell to the neighboring cell.
- During the passive uptake of salts, the ions are absorbed by the process of diffusion.
- The root hairs of plants absorb water from the soil by the process of diffusion.
- Osmosis is a special type of diffusion in which only the solvent molecules are allowed to diffuse.
- The Volatile aromatic compounds are present through the process of diffusion and this helps the nocturnal blooming flowers in attracting the moths for pollination.
What is Osmosis?
Osmosis is defined as the process of movement of solvent molecules from a region of high water potential to a region of low water potential while the two regions are separated by a semipermeable membrane.
The phenomenon of osmosis was discovered by Nollet and the term ‘osmosis’ was also coined by him.
All osmosis is diffusion but all diffusion isn't osmosis. Osmosis is a special type of diffusion.
Types of membrane
- Semi-permeable membrane:
A membrane that allows the passage of solvent molecules through it but doesn’t allow the passage of solute molecules. Examples: cell membrane, the white membrane of an egg, parchment paper, etc.
- Permeable membrane:
A membrane that allows the passage of solute and solvent is called a permeable membrane. Examples: cell wall, filter paper, etc.
- Impermeable membrane:
A membrane that doesn’t allow passage of solute and solvent is called an impermeable membrane. Examples: plastic sheet, rubber sheet, lignified and suberized cell wall, etc.
- Selectively permeable membrane:
A membrane that allows the passage of solvent molecules along with some of the selected solutes to pass through it. Examples: cell membrane.
Types of Osmosis
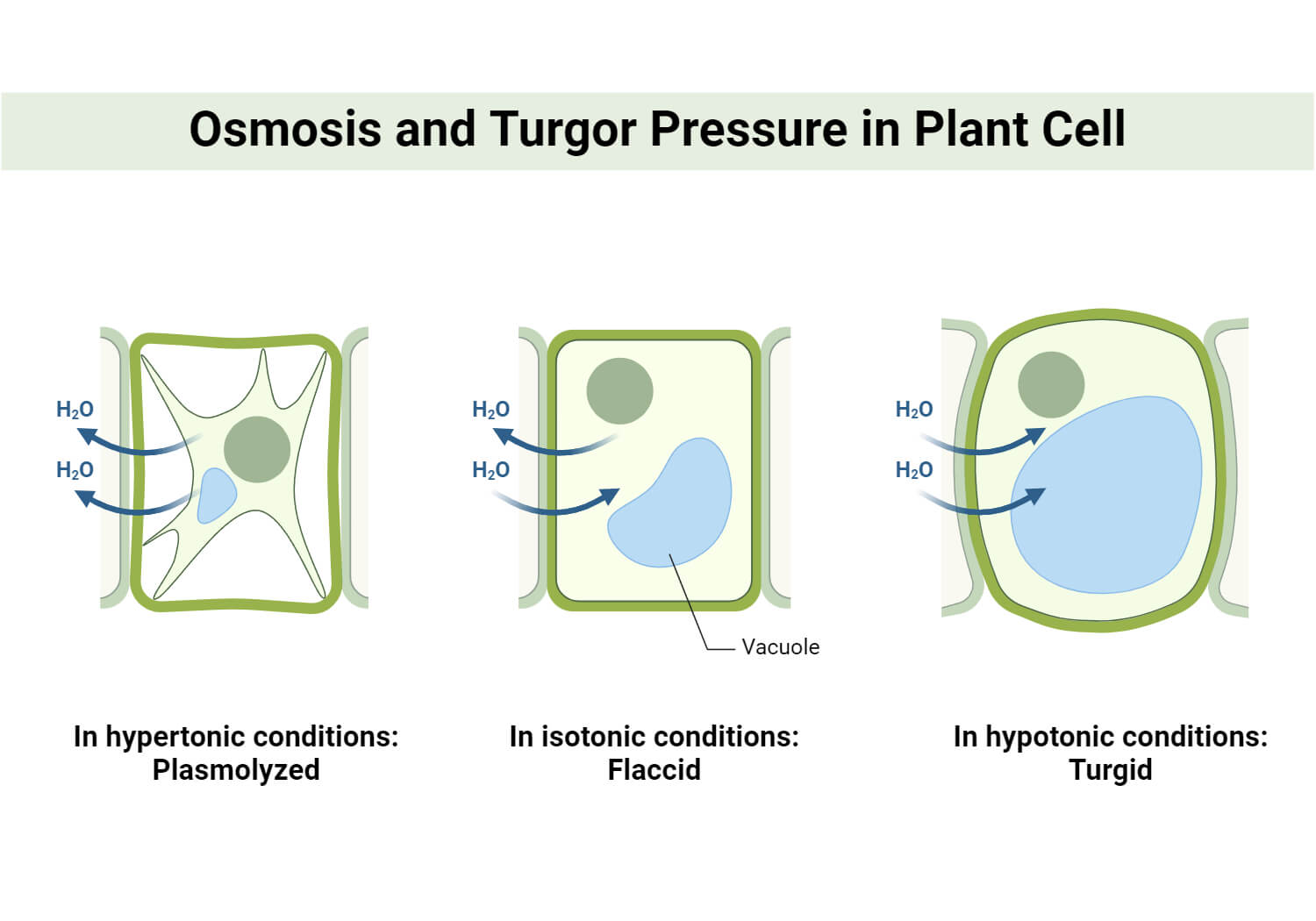
- Endosmosis:
It is the type of osmosis in which this is the entry of water into the cell when the cell is placed in a hypotonic solution. Example: When dried raisins are placed in water, the raisins swell up due to the entry of water into the raisin through the semipermeable skin of the raisin.
- Exosmosis:
It is the type of osmosis in which there is an exit of water when the cell is placed in a hypertonic solution. Example: When fresh grapes are placed in a high-sugar solution, water from the grape moves out into the sugar solution through the semi-permeable skin of the grape.
Types of Solution
- Hypotonic solution:
A solution whose concentration is lower than that of the cell sap of the cell.
- Hypertonic solution:
A solution whose concentration is greater than that of the cell sap of the cell.
- Isotonic solution:
A solution whose concentration is equal to that of the cell sap of the cell.
| Hypotonic solution | Hypertonic solution | Isotonic solution | |
| Plant cell | Water enters the cell and the cell becomes turgid. | Water moves out of the cell and the cell becomes plasmolyzed. | No change |
| Animal cell | Water enters the cell and the cell swells up and finally bursts. | Water moves out of the cell and the cell shrinks in size. | No change |
Fun fact: When human RBC is placed in distilled water, water enters the RBC and swells up to finally burst.
Osmotic Pressure
The excess hydrostatic pressure that should be applied to a solution to make it capable of stopping the further flow of solvent molecules into it through the semipermeable membrane is called the osmotic pressure of the solution. It is denoted by OP. It is measured in atmosphere or bar unit. It depends on salt concentration.
Water always moves from a region of low OP to a region of high OP. The osmotic pressure of pure water is zero. Hence, the osmotic pressure of a solution will always be greater than zero i.e. it will be positive.
Reverse Osmosis
It is the process of forcing the solvent molecules from a region of high solute concentration (high OP) to a region of low solute concentration (low OP) through a semipermeable membrane by applying an external pressure over the OP of the solution. It is a water purification technology that is used to obtain potable water from seawater. [The OP of seawater is +27 atm.]
Osmotic Potential
The Osmotic Potential of a solution is the potential of water molecules to move from a hypotonic solution to a hypertonic solution. It is represented by the Greek letter ‘π’. It is measured in kinetic energy terms. The osmotic potential of pure water is zero, hence, the osmotic potential of a solution will always be lesser than zero i.e. negative. Water always moves from a region of high π to a region of low π.
The osmotic potential and the osmotic pressure of a given solution have the same magnitude but have opposing coefficient signs.
Water Potential
The water potential of a solution is the solution’s capacity to give out water. It is represented by the Greek letter ‘ψ’. It is measured in bar units. [1 bar = 0.987 atm]
The water potential of pure water is zero. Hence, the water potential of any solution will always be less than zero. Water always moves from a region of high water potential to a region of low water potential.
The water potential of a solution is also related to its DPD as =-DPD
Osmotic Relations in a Plant cell
- Turgor Pressure
The outward pressure exerted by the fluid content of cell onto the cell wall is called turgor pressure. The inward pressure exerted by the stretched cell wall onto the cell content Is called wall pressure. At any time, the turgor pressure and wall pressure have the same magnitude but are opposite in direction. It is denoted by TP.
- Suction Pressure
The suction pressure of a cell is the cell’s capacity to take in water. It is denoted by SP. The suction pressure of a cell is related to the osmotic pressure and turgor pressure as follows:
SP = OP – TP
As water enters the plant cell, the turgor pressure of the cell increases while the osmotic pressure decreases and when the pressures are equal,
SP = 0
As the suction pressure of the cell becomes zero, water stops entering the cell. Hence, the plant cell never bursts when placed in a hypertonic solution or water. This filled condition of the cell is called ‘turgid’.
However, in a flaccid cell, the turgor pressure of the cell is zero. So,
SP = OP
As the suction pressure of a flaccid cell is equal to the osmotic pressure of the cell, the water-absorbing capacity of the cell depends on the solute concentration of the cell or the osmotic pressure.
Significances of Turgidity
- Turgidity helps in maintaining the shape and form of the plant.
- The turgid cells present at the tip of the root help in the penetration of the root into the soil.
- The leaves are held in a horizontal position by the tightly packed turgid cells of the leaves.
- The opening and closing of stomata are regulated by the change in turgidity of the guard cells.
- Turgidity is essential for cell division and is essential for proper growth and development of plants.
- The characteristic movement of the leaves of touch-me-not plant when touched is due to the change in the turgidity of leaf cells.
- The herbaceous plants with non-woody stems are held erect through closely packed turgid cells.
- Turgidity prevents plants from wilting.
Significances of Osmosis
- Water is absorbed by the process of osmosis in roots.
- Cell-to-cell movement of water is possible due to osmosis.
- Turgidity, resulting from osmosis, helps in maintaining the shape and form of the plant body.
- Osmosis affects the guard cell movement due to turgor changes and hence regulates the opening and closing of stomata.
- The rigidity of plants is maintained by turgidity, resulting from osmosis.
References
- A Brief About The Plant Physiology (byjus.com)
- Plant-Physiology-by-Vince-Ordog.pdf (usp.br)
- Advances in Selected Plant Physiology Aspects – Google Books
- 39.1: Systems of Gas Exchange – The Respiratory System and Direct Diffusion – Biology LibreTexts
- Tracing the flow of carbon dioxide and water vapor between the biosphere and atmosphere: A review of optical isotope techniques and their application – ScienceDirect
- 5.6: Passive Transport – Diffusion – Biology LibreTexts
- 10.26: Osmotic Pressure – Chemistry LibreTexts
- Endosmosis And Exosmosis – The Major Differences (byjus.com)
- Chapter 7 Evaluation of Turgidity, Plasmolysis, and Deplasmolysis of Plant Cells – ScienceDirect
- Effect of potassium on the water potential, the pressure potential, the osmotic potential and cell elongation in leaves of Phaseolus vulgaris – Mengel – 1982 – Physiologia Plantarum – Wiley Online Library
