Definition of Anomalous Expansion of Water
The “anomalous” term used here means the unusual as expansion of water shows the unusual behavior of as it cools between 4 °C and 0 °C. Basically most of the substances contract upon cooling while water expands in this temperature range. This weird behavior questions the common idea that a substance will become denser as it cools. In most cases, fluids grow thicker as the temperature drops. This is because their molecules migrate closer together due to decrease in kinetic energy. Water, on the other hand, has a unique quality of hydrogen bonding giving less dense structure.
This phenomena is essential in observing many natural processes. It is one of the most researched features in physics and chemistry. When water reaches 4 °C, it is most dense but continues to expand as soon as the temperature falls. Hence it freezes at 0°C showing the least density. Finally, the solid form seen after freezing (ice) starts floating.
This action is referred to as “anomalous” since it violates the natural phenomenon that materials get denser as they cool. Understanding this feature is crucial in every science field. Our everyday applications are also influenced by this feature of water.. It helps to explain occurrences such as icebergs floating and water pipelines exploding in freezing temperatures.
Molecular Basis: Hydrogen-Bond Network and Ice Lattice
At the molecular level, the hydrogen bonding is the major reasoning for the explanation of water’s unusual behavior. Water molecules develop negative charge near the oxygen atom and a positive charge near the hydrogen atom which allows them for bond formation. This bond formation occurs when both these charges attract each other. At temperatures above 4 °C, the molecules move faster so that the formed bonds break down or disappear. On the other hand, as water cools below 4 °C, the kinetic energy of molecules slows down and hydrogen bonds start controlling the molecules.
As a result, water molecules organize in a hexagonal lattice structure like ice. At higher temperatures, the molecules are located farther apart than they are in liquid condition. As a result, water expands as it cools below 4 °C due to the additional volume occupied by this lattice.
When water freezes, the crystal lattice becomes even more stiff and open which has larger volume and a lower density. This particular structure is responsible for the floating of ice on water.
Maximum Density at 4 °C
The ultimate density of water is gained at 4 °C. Above and below this temperature, water starts losing its density. The chemical reaction resulting from the kinetic energy of water molecules contributes to hydrogen bonding. This finally results in solid crystalline structures of the molecules.
When temperature goes above 4 °C, the water molecules increase their pace. This causes a slight increase in its volume but the density lowers. In contrast, below 4 °C, the formation of the hydrogen-bond allows the water molecules to shift their phase where the volume is again increased, while the density is decreased.
This maximum density is not only a value but a region for environmental research and aquatic ecosystems. In natural bodies of water such as lakes and ponds, the densely packed water falls to the bottom and provides a layer which protects the water under it. This provides a suitable environment for aquatic life. The behavior of water at 4 °C is extremely important in laboratory and industrial processes where this density is tightly maintained. It also has significant importance on tools and structure constructions which work under temperature-sensitive cases.
Why Ice Floats: Lower Solid-Phase Density
One of the most evident observations of this anomalous expansion is the floating of ice on water. The density of water lowers when in a frozen state than in its liquid state. This is probably due to the crystalline form of ice, which is bonded together by hydrogen and hence permits water molecules to set free more than in liquid form. Each water molecule makes stable hydrogen bonds with four neighbouring molecules, which gives an open hexagonal structure. The configuration takes up more area than the unorganized, tightly packed structure of liquid water. As a result, ice has a lesser density and floats.
This property of water seems extremely important for biological survival.. When lakes or oceans freeze during extremely cold weather, ice floats on their surfaces. This ice acts as a blockade for heat flow and preserves the temperature below. This safeguards aquatic life from extreme cold. The fact that ice floats influences human behavior. Since icebergs float with only a small portion above the water, its orientation can give an illusion and cause major accidents while travelling on ships and boats. Engineers must also consider this feature while constructing designs for cold weather, to avoid the harmful aspects of freezing water.
Survival of Aquatic Life in Winter
The anomalous expansion of water plays a significant role in the protection of aquatic life during the winter. As the temperature falls in winter, the surface water cools and shrinks. Thus it goes towards the bottom. After reaching 4°C, its density again increases and becomes denser than that of water at 0°C. This causes it to go to the bottom layer. In comparison, water at 0°C becomes less dense and remains on the top layer. After freezing the ice is formed and keeps floating upward. Fish and other aquatic species can easily survive in this layer of temperature throughout the winter. Without this phenomena, entire ecosystems would be harmed by cold temperatures.
Furthermore, the ice covering on the surface works as an insulator that minimizes heat loss from the water underneath and reduces the passage of freezing air. It also blocks oxygen exchange with the atmosphere, which can have an effect on aquatic life; however, many species are habituated to these variations in the season.
This natural protecting effect helps biodiversity and environmental stability in colder climates. It also highlights the importance of water’s unique thermal qualities in keeping organisms alive, particularly in polar and transitional situations.
Freeze-Thaw Weathering and Soil Formation
The anomalous expansion of water can affect the geology of earth which is known as freeze-thaw weathering. This event frequently shows up when water enters the cracks in rocks and then freezes. As water freezes, it expands by putting ultimate pressure on the surrounding rock. Continuous freezing and thawing results in the breaking down of the granite.
Freeze-thaw erosion helps greatly to the mechanical decay of rocks in cold climates and mountainous areas. It is essential for soil formation because breaking down rock pieces combine with organic substances to make fertile topsoil. As time passes, this contributes to the evolution of landforms and ecosystems.
This type of degradation in rocks must be considered while designing any architecture. In colder places, freeze-thaw cycles can damage roads, bridges, and buildings. Engineers must consider this while constructing structures, applying materials and methods of construction that reduce water penetration and allowing expansion pressures.
Also, freeze-thaw cycles affect agriculture, degradation patterns, and the movement of sediment. Understanding this process helps in identifying geological changes and managing the use of land in prone areas. It also demonstrates the deeper importance of water’s remarkable extension outside ecosystems and into the mechanical makeup of the Earth.
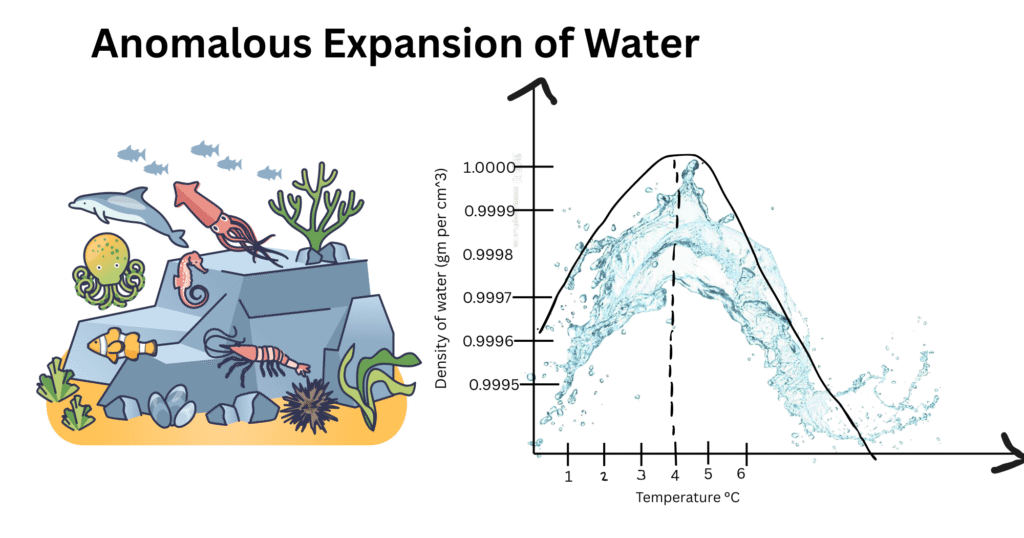
Density vs. Temperature Graph (0 – 10 °C)
A density versus temperature graph is drawn for water for the temperature range between 0 °C to 10 °C. This helps to demonstrate the unusual behavior of water. Here, the y-axis represents the density of water, while the x-axis represents the temperature in Celsius scale. As the temperature increases from 0 °C, the graph also rises until it reaches 4 °C. Beyond this point, the graph starts to descend with increasing temperature.
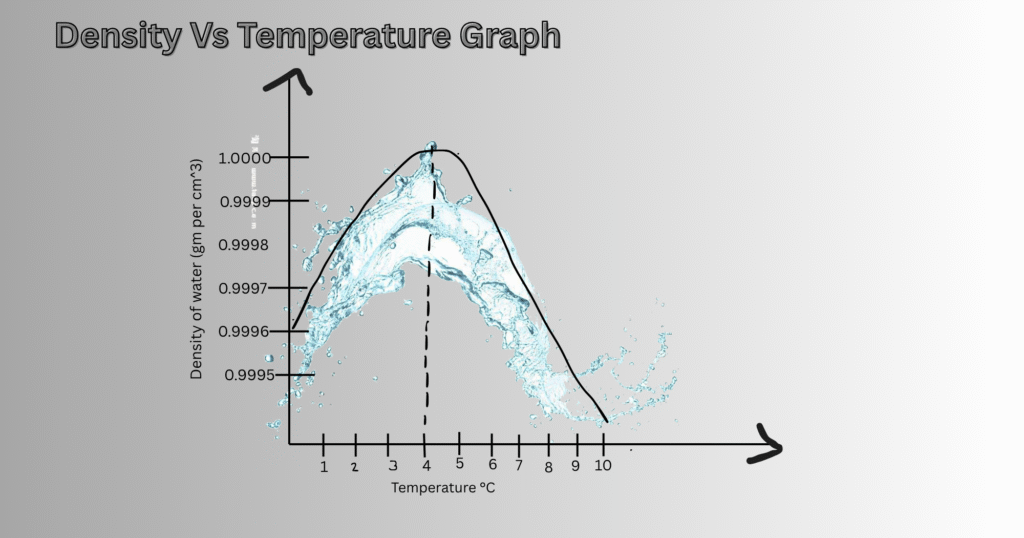
This non-linear relationship is in contrast to most other substances, whose density decreases with increasing temperature. The graph-curve is concave near 4 °C and marks the point at which water flips from expansion to melting when heated.
This graphical representation is important for understanding water’s physical behavior and depicting its optimum density. Scientists apply these facts for simulations of ecological structures, conduct experiments, and examine environmental events.
The graph also has significance for limnology (the study of inland waters), where it helps to understand the formation of thermal layers in lakes. It is an essential concept in hydrology, climatology, and environmental science, used for predicting water behavior in both natural and artificial environments.
Laboratory Demonstrations: Hope’s Apparatus
A Scottish chemist Charles Hope brought up the apparatus which shows how water behaves differently near 4 °C. It’s a tall, cylindrical container with a cooling jacket around its center.
To carry out the experiment, the cylinder is filled with water and thermometers are placed at various heights to measure temperature changes. To cool the water at the center, the jacket is filled with ice or a cooling agent. As the water cools, a decline in temperature is noticed in the thermometer. This shows up until it becomes 4°C. At this point, the water at that level goes down to the bottom due to its high density. When the temperature is lowered below 4 °C, the less dense water begins to rise, showing unusual behavior.
Hope’s apparatus is used extensively in lab demonstrations of physics and chemistry education. This provides an accurate visual picture of the density maximum at 4 ° C. It also helps students and researchers understand the practical implications of theoretical concepts and confirms the experimental evidence for this anomalous expansion.
This demonstration is not only educational but also serves as the foundation for topics such as molecular behavior, hydrogen bonding, and the practical applications of water’s features.
Engineering and Everyday Applications
Water’s anomalous expansion has an impact on many kinds of engineering applications and also some common issues. In a cold climate, the expansion of water can harm the enclosing structures and require expensive maintenance. Engineers must consider these characteristics while designing pipes, such for employing insulating materials.
In a construction, the base must be designed to resist freeze-thaw cycles caused by water seepage. This process can potentially cause damage to roads and bridges. Materials are chosen by considering their capacity to endure the stress produced by freezing water.
On a daily basis, this property describes why water bottles usually crack when stored in the freezer. In laboratories, precise measurements of water density are required for calibration and experimentation. Devices like hydrometers rely on accurate density readings that have to account for the unusual behavior of water.
This characteristic is also useful in climate control systems, water storage, and agricultural irrigation planning. Understanding and managing water’s anomalous expansion is critical for reliability, effectiveness, and efficiency in a variety of sectors.
Comparison with Normal Thermal Expansion in Other Liquids
Most liquids show a universal pattern of expanding on raising the temperature and contracting on lowering it. This behavior is due to the rise in their kinetic energy of molecules with the temperatures. This forces them to move away from each other. Conversely, reduced temperature gives densely packed molecules.
However, water denies this pattern near its freezing point. Between 4 °C and 0 °C, it shows an expansion rather than contraction.This is probably due to the formation of a more arranged hydrogen-bond at this temperature. Other liquids like ethanol, mercury, and oils do not show such weirdness. Their density-temperature graph shows a smooth curve without any discrepancy.. Thus the distinct characteristics of water suggest its own unique molecular structure which is critical for the Earth’s biosphere.
The difference between water and other liquids is often utilized in educational fields to show the importance of molecular bonds. It also has practical applications in industries where fluid behavior at different temperatures must be precisely planned and managed.
Understanding these variations helps in the creation of more effective cooling systems, accurate thermometers, and precise scientific instruments.
Influence on Lakes, Oceans, and Climate Moderation
In lakes and oceans temperature cycles are impacted by the unusual expansion of water.. This helps to regulate their climate. The formation of thermal strata occurs in lakes when the maximum density is reached at 4 degrees Celsius. During the winter, the solid but lighter ice stay on top, while more warm water remains at the bottom. This layering helps maintain the aquatic environment.
Although salinity and pressure actively impact the oceans, water’s anomalous feature persists in action. Bottom surface of the seas are protected by the ice formation on the top layer, which prevents them from freezing completely and protects marine life. This thermal action also helps in maintaining the Earth’s climate. Water’s high specific heat capacity and expansion features enable it to absorb and release huge amounts of heat with small temperature changes. This adjusts atmospheric temperatures and minimizes extreme weather.
Furthermore, the floating ice reflects sunlight, which contributes to the Earth’s reflectance and helps to keep the balanced energy of earth. Thus, water’s unusual behavior is not simply a physical and chemical perplexity; it is an essential part of the Earth’s climate system, influencing everything from local ecosystems to global climate equilibrium.
Conclusion
The anomalous expansion of water is a surprising but a major crucial physical property with global implications. It is a bit complex but a significant phenomenon originating from hydrogen bonding. Its great positive impacts can be seen on climate, ecosystems, engineering, and daily life.
Its major role in the possibility of aquatic life, erosion of rocks, soil formation, and climate stability reveals how even small molecules can have an enormous effect on the whole planet. Understanding this phenomenon extends our understanding of natural processes and encourages for the advancements in science and industry.
Finally, the anomalous expansion of water works as a notable signal to depict the remarkable impact of how simply linked physical and chemical compositions of materials can alter nature. (Also read about Floatation)
References
Nilsson, A., & Pettersson, L. G. (2015). The structural origin of anomalous properties of liquid water. Nature communications, 6(1), 8998.
Everett, D. H., Haynes, J. M., & McElroy, P. J. (1971). The story of anomalous water. Science Progress (1933-), 59(235), 279-308.
Errington, J. R., & Debenedetti, P. G. (2001). Relationship between structural order and the anomalies of liquid water. Nature, 409(6818), 318-321.
https://byjus.com/physics/properties-of-water-anomalous-expansion-of-water/
https://unacademy.com/content/cbse-class-11/study-material/physics/the-anomalous-behaviour-of-water/
