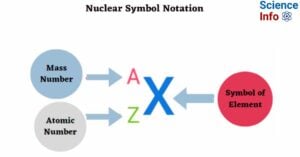
Hydrogen Embrittlement: Causes, Types, Prevention
Hydrogen embrittlement is a loss of ductility caused by excessive hydrogen absorption, rendering the material brittle. This is because hydrogen atoms are substantially smaller than those that make up the deposited … Read more