The term “density” refers to how much volume (space) an object or substance takes up to the amount of material it contains (mass). Density, or the quantity of mass per unit volume, is one of the unique characteristics of water. In many scientific fields, such as chemistry, physics, and engineering, an understanding of the density of water is crucial. The weight of water per unit volume is known as its density, and it is influenced by the water’s temperature. The density of pure water is 1 gm/cm3 or 1000 kg/m3. At 4 °C, the density of water reaches its maximum.
Interesting Science Videos
What is Density?
Density is defined as a material’s mass per unit volume. Density is the ratio of mass to volume or mass per unit volume. Density is essentially a measure of how closely matter is packed together. Archimedes, a Greek scientist, devised the principle of density, which is simple to calculate if you know the formula and its units.
The density of an object is typically expressed by the Greek letter “ρ”. To calculate it, take the mass (m) and divide it by the volume (v):
ρ = m / v
The SI unit for density is kilogram per cubic meter (kg/m3). It is also usually expressed in the cgs unit of grams per cubic centimeter (g/cm3).
What is Density of Water?
The density of water is the weight of the water per its unit volume.
The density of water at normal temperature is 998.2 kg/m3. The standard calculating unit is 1 gram per milliliter (1 g/ml) or 1 gram per cubic centimeter (1 g/cm3). While you can round the density to one gram per milliliter, there are more accurate numbers to consider. Pure water has a density of little less than 1 g/cm3. Water can be supercooled and remain a liquid far beyond its regular freezing point. Water has a maximum density of around 4 degrees Celsius. Ice has a lower density than liquid water, hence it floats.
Air pressure and atmospheric temperature also influence the density of water. However, because these density variances are quite minor, you can continue to use 1 g/cm3 for water density unless you need to perform highly detailed calculations or the experiment is carried out under extreme temperatures or pressures.
The density of water in various metric systems is provided below:
| Unit System | Density of Water |
| g/cm3 | 1 g/cm3 |
| g/mL | 1 g/mL |
| kg/m3 | 1000 kg/m3 |
| lb/ft3 | 62.4 lbs/ft3 |
Formula of Density of Water
The density of water is determined using the normal density formula.
D = M/V
Where
- D is the density of water.
- M is the mass of water.
- V is the volume of water.
We know that.
At room temperature, the mass of one milliliter of water is 0.995 grams. Now, the density of the water is
D = Mass/Volume
0.995/1 = 0.995 g/ml = 0.995 g/cm3.
Factors Affecting Density of Water
The density of a substance can be affected by a variety of conditions. Some of the factors affecting water density are given below:
- The density of water is approximately 1 gram per cubic centimeter (1 g/cm3).
- Although the relationship is classified as non-linear and unimodal rather than monotonic, it is temperature-dependent.
- Liquid water tends to become denser when chilled from room temperature, however, it is believed that pure water reaches its maximum density at around 4 °C and that the density of water increases from 4 °C to 0 °C, a phenomenon known as the anomalous behavior of water.
- As it cools, it tends to expand and lose density. Thus, water is an unusual liquid that becomes denser to a certain degree when cooled and then begins to expand as it cools further.
Density of Water at Different Temperature Scales
Water does not have an absolute density since its density changes with temperature. It is denser in the liquid form than in the solid.
The density of water at room temperature, which is between 20 and 25 degrees Celsius, is 0.9982 g/cc, or 998.2 kg/m3. At room temperature, water’s mass is almost equal to its volume, and it remains liquid. Seawater has a slightly higher density than regular water because it contains various salts and minerals. Seawater typically has a density of around 1027 kg/m3.
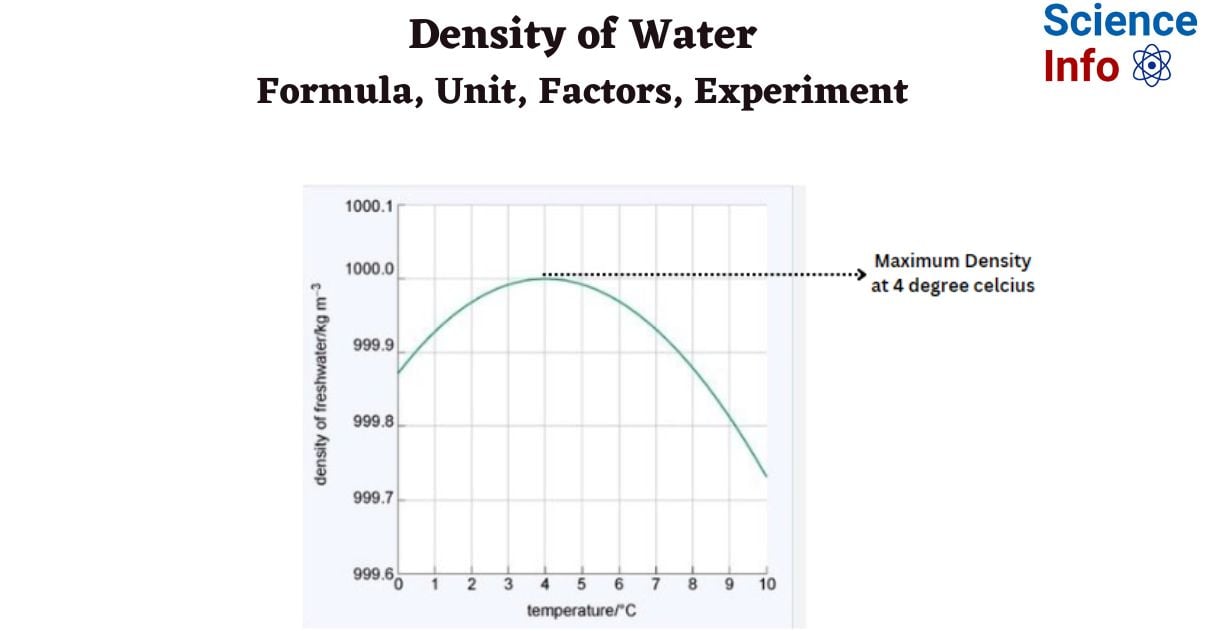
Check out the Density vs. temperature graph below to see how density varies with temperature.
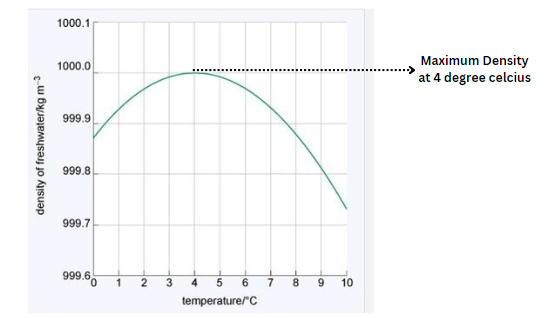
The density of water fluctuates depending on temperature. The density (in kg/m3) of water at various temperatures (ranging from -30°C to 100 °C) is shown in the table below.
| Temperature (°C) | Density (kg/m³) |
| -30 | 983.854 |
| -20 | 993.547 |
| -10 | 998.12 |
| 0 | 999.83 |
| 4 | 998.97 |
| 10 | 999.70 |
| 15 | 999.1 |
| 20 | 998.2 |
| 22 | 997.77 |
| 25 | 997.04 |
| 30 | 995.65 |
| 40 | 992.2 |
| 60 | 983.2 |
| 80 | 971.8 |
| 100 | 958.4 |
Experiment on Density of Water
To explore the density of water and its comparison with other substances, we can conduct a simple experiment. Here’s how:
Step 1: Gather a tall glass cup, honey, water, coconut oil, and food coloring.
Step 2: Begin by pouring a one-quarter cup of honey into the glass.
Step 3: Gently pour a one-quarter cup of colored water on top of the honey.
Step 4: Finally, pour a one-quarter cup of coconut oil on top of the colored water.
Observe how each substance settles in distinct layers within the glass. This illustrates the concept of density, wherein substances of different weights occupy different positions based on their densities. Heavier substances like honey sink to the bottom, while lighter ones like oil float at the top.
Additionally, for a more comprehensive understanding of water’s density, we can conduct another experiment:
Step 1: Put small volumes of viscous liquids such as liquid soap, honey, ethyl alcohol, olive oil, and water in a test tube.
Step 2: Mix the liquids thoroughly to ensure they are evenly distributed.
Step 3: Allow the test tube to lie undisturbed for a few hours, allowing each component to separate into discrete layers based on density.
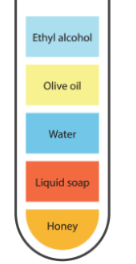
Upon examination, it becomes clear that each substance has a distinct density, resulting in the creation of layers within the test tube. Heavier chemicals sink to the bottom, while lighter ones rise to the top. This experiment demonstrates how the density of several substances, including water, affects their behavior and location relative to one another.
Relative Density of Water
Relative density, or specific gravity, is the ratio of a substance’s density to that of a certain reference material. Specific gravity is almost always measured for water at its densest point (4°C) for liquids and air at room temperature (20°C) for gases. So, water has a relative density of 1.0. If the substance is water, its relative density will be one because we are comparing the same things.
If a substance’s relative density is less than one, it is less dense than the reference. If it is larger than one, it is denser than the reference. The densities are equivalent if the relative density is exactly one, which means that equal volumes of both substances have the same mass. If water is chosen as the reference material, any substance with a relative density lower than one will float in it. A material with a relative density higher than one sink.
Relative Density = Density of the substance/ Density of water
Relative density is a dimensionless quantity that represents the ratio of densities.
Anomalous Behaviour of Water
Liquids at moderate temperatures expand when heated and contract when cooled. Water, on the other hand, acts unusually. The general tendency of cold water remains constant until 4 degrees Celsius. Water gradually becomes denser as it cools. The density of it reaches its maximum at 4oC. Water expands with a further drop in temperature, so when you cool it from 4oC to 0oC—which is when you want to generate some ice—the density of the water reduces.
This water expansion has the effect of keeping the coldest water near the surface at all times. The heaviest water, or that which is 4 oC, sinks to the bottom of the body of water, while the lightest water, or that which is coldest, builds up on the top layer. Consequently, the water’s surface always freezes over first in the winter months. Because both water and ice are poor heat conductors, the top layer of ice shields the remaining portion of the body of water from the winter’s chill, safeguarding all of the wildlife therein.
Reason for Anomalous Behaviour of Water
Two hydrogen atoms and one oxygen atom make up a water molecule. Because of their intermolecular interaction, water molecules stay together in a liquid form at room temperature. Water molecules are always traveling around in the container and rearranging themselves when they are in a liquid state.
Hydrogen contributes to intermolecular attraction. Another feature of water molecules is the attraction between the hydrogen atoms of one molecule and the oxygen atoms of another. The O-O attraction in water is less than the distance between the H-O bonds. The molecules in cooling water move less quickly as their energy is dissipated. As it cools down even further, the molecules of water begin to compress together, becoming denser. The water molecules can no longer compress past the density’s maximum at 4oC.
Therefore, it is the H-O attraction rather than the O-O attraction that keeps the water from freezing over into ice. Water molecules are unable to migrate because of the ice’s lattice structure. However, as the H-O bond takes over, it expands slightly since it is not quite as tight as the O-O connection.
Frequently Asked Questions (FAQs)
What is the unit for density?
The unit of density is generally measured in grams per cubic centimeter or g/cm3. Density is measured in standard units, such as kilograms per cubic meter (kg/cm3).
What is the symbol of density?
Density is commonly represented by the symbols “ρ” and “D.”
At what temperature does water have its maximum density?
Water has a maximum density of 4°C, or 39°F.
Why does ice float in water?
The density of water reaches its maximum at 4°C. Since ice is less dense than liquid water, it floats. The density of the ice drops by roughly 9% as it freezes.
What is the density of water at room temperature?
At room temperature, water has a density of 998.2 kg/m3.
What is the density of seawater?
Seawater has an approximate density of 1027 kg/m3 at the surface.
Video on Density of Water
References
- https://infinitylearn.com/surge/blog/neet/important-topic-of-physics-anomalous-expansion/
- https://www.usgs.gov/special-topics/water-science-school/science/water-density#
- https://www.geeksforgeeks.org/density-of-water/
- https://testbook.com/chemistry/density-of-water
- Helmenstine, Anne Marie, Ph.D. “What Is the Density of Water?” ThoughtCo, Oct. 24, 2022, thoughtco.com/what-is-the-density-of-water-609413.
- https://byjus.com/physics/properties-of-water-anomalous-expansion-of-water/#why-does-it-happen

