Endergonic and exergonic are two types of chemical reactions, or processes, in thermochemistry or physical chemistry. The names describe what happens to energy during the reaction. The key difference between endergonic and exergonic is that endergonic reactions are non-spontaneous and unfavorable, whereas exergonic reactions are spontaneous and favorable.
Interesting Science Videos
Endergonic Reaction
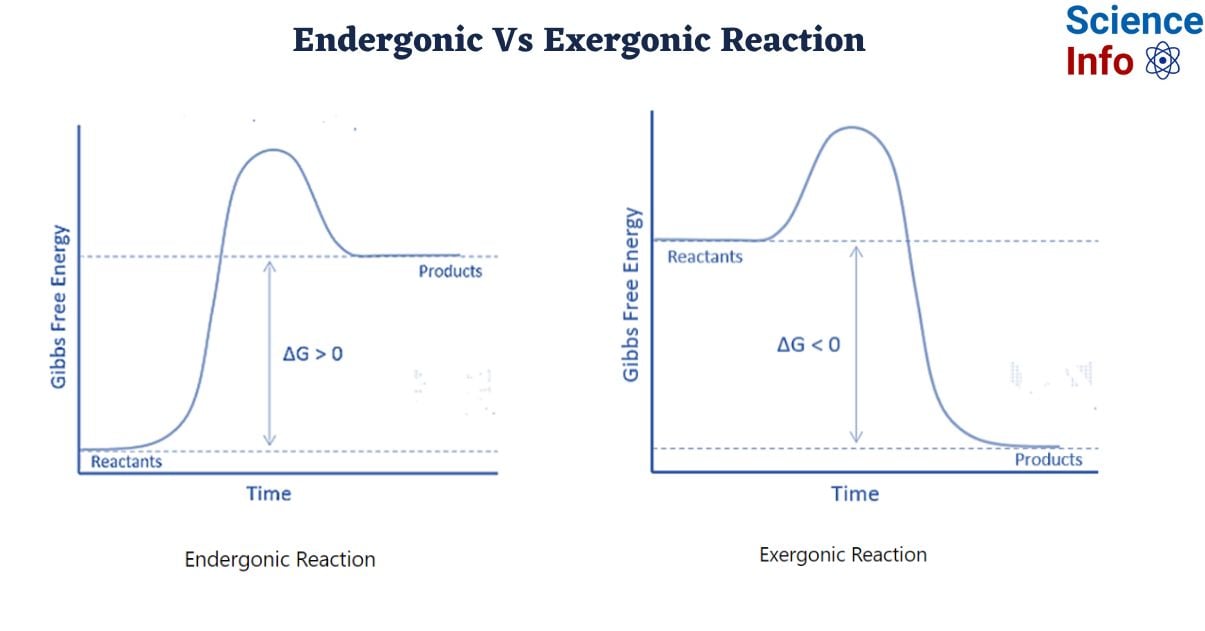
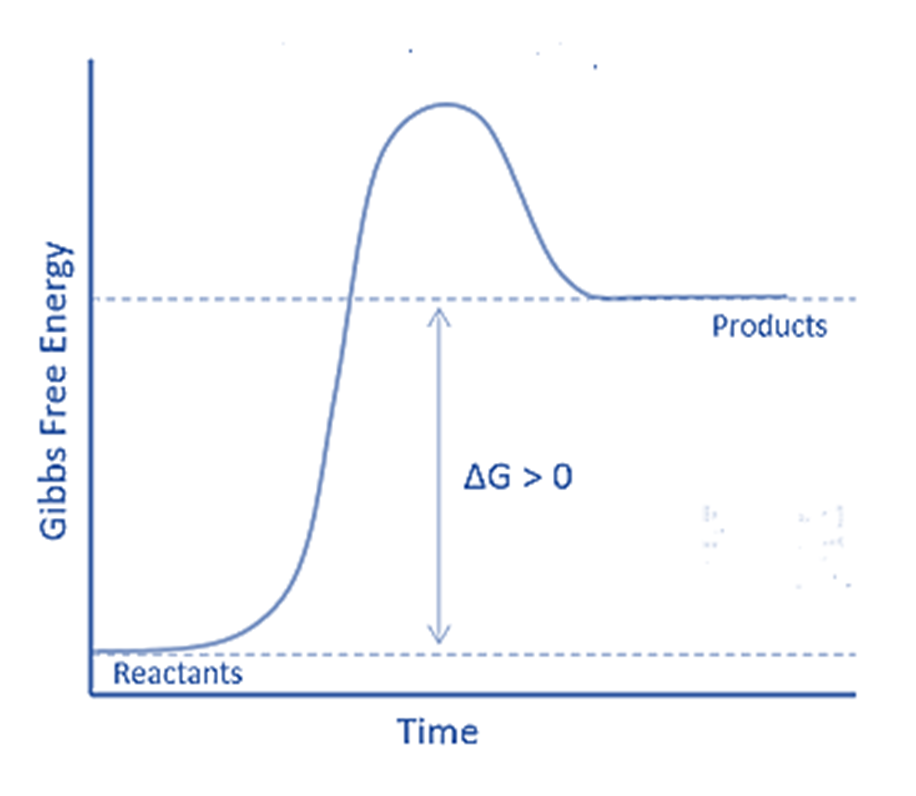
The word “ender” comes from the word “endo,” which meaning “within”. Thus, endergonic refers to the absorption of energy in the form of work. As a result, in an endergonic reaction, the system receives energy from its surroundings. Furthermore, the products will contain more energy than the reactants. An endergonic reaction is characterized as nonspontaneous or unfavorable. If this energy transfer occurs under constant pressure and temperature, the standard Gibbs free energy will be positive. Thus, the equilibrium constant of an endergonic reaction is smaller than one.
Endergonic reactions form new chemical bonds that store energy until they are broken, releasing the energy. However, the newly established chemical bonds are weaker than the previously broken ones. The endergonic reaction tries to synthesize building blocks of life, such as DNA and RNA.
Examples of Endergonic Reactions
- Photosynthesis: Photosynthesis is an endergonic reaction because plants use the sun’s energy to turn carbon dioxide (CO2) and water (H2O) into sugar (C6H12O6) and oxygen (O2)
6 CO2 (g) + 6 H2O (l) + energy → C6H12O6 (s) + 6 O2 (g)
- Melting: Water melts into ice by absorbing heat from its surroundings.
H2O (ice) + energy → H2O (water)
- Water electrolysis: An electric current is used to supply energy to water (H2O), resulting in the formation of hydrogen (H2) and oxygen. This technique, known as electrolysis, is a redox reaction.
2 H2O (l) → 2 H2 (g) + O2 (g)
- Formation of nitrogen monoxide: At normal to high temperatures, nitrogen (N2) and oxygen (O2) in the atmosphere mix to produce nitrogen monoxide or nitric oxide (NO).
N2 (g) + O2 (g) → 2 NO (g)
- Other examples are DNA/RNA synthesis, protein synthesis, and fatty acid synthesis. Furthermore, a polymerization reaction is endergonic since it decreases entropy.
Exergonic Reaction
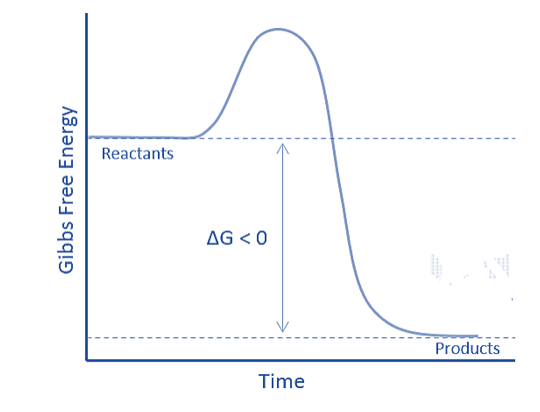
Exergonic means releasing energy in the form of work. These reactions transfer energy from the system to the outside. Exergonic reactions are favorable and occur spontaneously. Because energy is released throughout the reaction, the products have lower energy than the reactants. The enthalpy change (∆H) becomes negative. Furthermore, if the transfer is carried out at constant pressure and temperature, the standard Gibbs free energy will be negative. For example, an exergonic reaction is a chemical reaction having a negative standard Gibbs free energy at constant temperature and pressure: ∆G° < 0.
Exergonic reactions, like all other reactions, require activation energy to proceed. However, the energy generated by the reaction is sufficient to match the activation energy and keep the reaction going. While an exergonic reaction is spontaneous, it may take longer to complete without the need of a catalyst. For example, the corrosion of iron is exergonic yet extremely slow.
Examples of Exergonic Reaction
- The combination of sodium (Na) with chlorine (Cl) to produce sodium chloride (NaCl).
2 Na + Cl2 → 2 NaCl + energy
- Hydrolysis of adenosine triphosphate (ATP) into adenosine diphosphate (ATD)
ATP + H2O → ADP + Pi + energy
- The combustion of propane (C3H8) with oxygen (O2),
5 O2 + C3H8 → 4 H2O + 3 CO2 + energy
Difference Between Endergonic and Exergonic Reaction
Endothermic and exothermic reactions are classified as endergonic and exergonic reactions, respectively. The difference is that heat is produced when an endothermic process absorbs energy or when an exothermic reaction releases energy. In addition to heat, endergonic and exergonic processes can produce light and sound. For example, a glow stick is an exergonic process that emits light. It is not an exothermic process because it produces no heat.
The major differences between endergonic and exergonic reactions is that the former are non-spontaneous and unfavorable, whilst the latter are spontaneous and favorable. Endergonic reactions, unlike exergonic reactions, will have a positive standard Gibbs free energy. Exergonic reactions produce products with less energy than the reactants, whereas endergonic reactions produce products with more energy.
| Categories | Endergonic Reactions | Exergonic Reactions |
| Definition | An endergonic reaction is a chemical reaction having a positive standard Gibbs free energy, at constant pressure and temperature. | An exergonic reaction is a chemical reaction with a negative standard Gibbs free energy, at constant temperature and pressure. |
| Energy Requirement | Endergonic reactions require an input of energy to proceed. They absorb energy from their surroundings. | Exergonic reactions release energy into their surroundings. They release more energy than they absorb. |
| Free Energy Change | The change in free energy (Δ𝐺) for endergonic reactions is positive (Δ𝐺>0). This means that the products have more free energy than the reactants. | The change in free energy (Δ𝐺) for exergonic reactions is negative (Δ𝐺<0). This means that the reactants have more free energy than the products. |
| Spontaneity | Endergonic reactions are non-spontaneous under standard conditions. They do not occur without an external source of energy. | Exergonic reactions are spontaneous under standard conditions. They can occur without an external source of energy. |
| Entropy | Entropy is decreased. | Entropy is increased. |
| Energy | In endergonic reactions, the products have more energy than the reactants. | In exergonic reactions, the reactants have more energy than the products |
| Endothermic and exothermic reaction | Endothermic reactions are endergonic | Exothermic reactions are exergonic. |
| Examples | Photosynthesis in plants is an example of an endergonic reaction. Plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen. | Cellular respiration is an example of an exergonic reaction. Cells break down glucose into carbon dioxide and water, releasing energy that is used to produce ATP. |
References
- https://www.differencebetween.com/difference-between-endergonic-and-vs-exergonic/
- https://www.chemistrylearner.com/chemical-reactions/endergonic-reaction
- https://sciencenotes.org/endergonic-vs-exergonic-reactions-and-examples/
- Helmenstine, Anne Marie, Ph.D. “Endergonic vs Exergonic Reactions and Processes.” ThoughtCo, Apr. 5, 2023, thoughtco.com/endergonic-vs-exergonic-609258.
- https://chemistrytalk.org/exergonic-and-endergonic-reactions/
- https://www.dictionary.com/compare-words/endergonic-vs-exergonic
