The nuclear symbol notation is a sort of shorthand expression that identifies the element’s symbol or atomic number as well as its mass number. Symbols are used to represent nuclei in the same way as chemical formulas and equations are. The complete nuclear symbol includes the symbol for the element as well as numbers indicating the number of protons and neutrons in that nucleus. Nuclear symbol notation is used for indicating the isotopes of elements.
Interesting Science Videos
What is Nuclear Symbol Notation?
Nuclear notation, commonly known as isotope notation, is a sort of shorthand notation that identifies an element (by symbol or atomic number) as well as its mass number.
Nuclear symbol notation or isotope notation is important because, instead of using a visual representation that requires a lot of words, it allows us to quickly compute the mass number, atomic number, and number of protons and neutrons in the nucleus of an isotope.
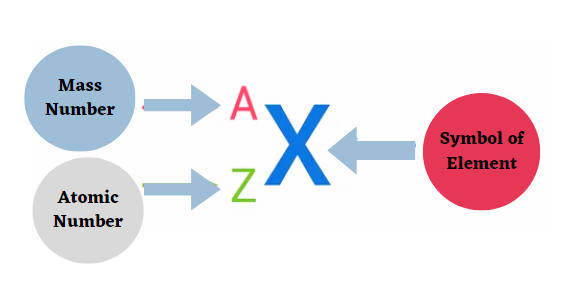
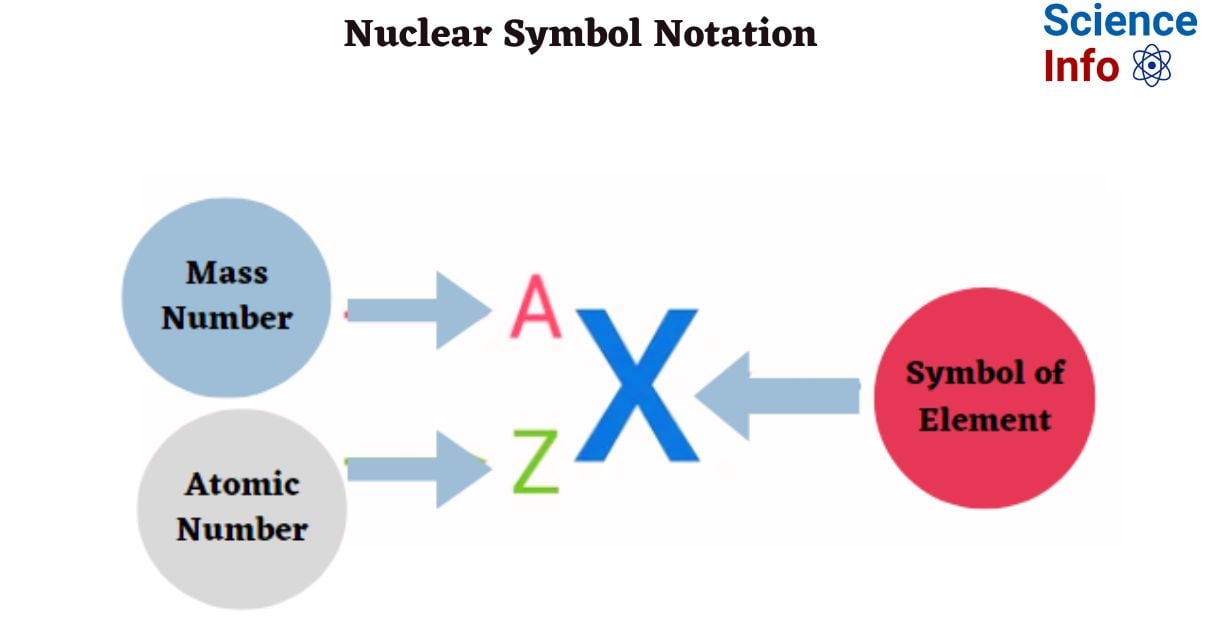
- Nuclear symbols can be written in two ways: the traditional method, which uses the element name or symbol followed by a dash and the mass number (e.g., carbon-14 or C-14), and the AZE or AZX notation, which uses the mass number as a superscript and the atomic number as a subscript before the element symbol (for example, 14C or 12C).
- In AZE or AZX notation:
A = mass number or the number of nucleons (protons and neutrons).
Z = atomic number, or the number of protons.
X = element symbol.
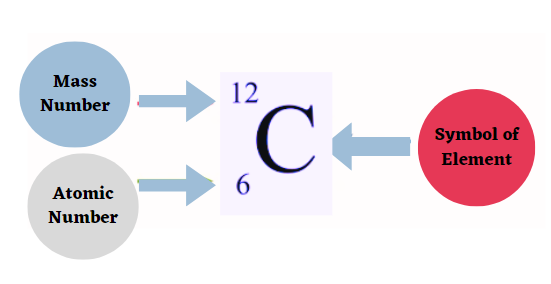
The nuclear symbols for the isotopes carbon-14 and carbon-12 are 146C and 126C, respectively, according to the AZE/AZX notation.
- The number of electrons is not included in the nuclear symbol because they are not part of the nucleus.
- Isotope ions, on the other hand, can be represented by nuclear symbols followed by a superscript indicating the electric charge. For example, 11H+ represents a protium ion (hydrogen-1) with a +1 electrical charge.
Let us now discuss some of the terms used in nuclear symbol notation, such as isotopes, mass numbers, and atomic numbers.
Isotopes
Atoms with the same atomic number but differing mass numbers because of a difference in the number of neutrons they hold are called isotopes. There could be two or more isotopes that have the same atomic number.
- Hydrogen has three naturally occurring isotopes: protium: 1H (with zero neutrons), deuterium: 2H (with 1 neutron), and tritium: 3H (with 2 neutrons).
- Carbon is an isotope-containing element. Carbon has three isotopes: 12C (with six neutrons), 13C (with seven neutrons), and 14C (with eight neutrons).
Mass Number
The mass number of an atom is determined by the number of protons and neutrons.
It is symbolized by the letter ‘A.’
- Together, protons and neutrons are referred to as nucleons since they are both found in an atom’s nucleus.
- An atom of carbon, for instance, consists of six protons and six neutrons. Its mass number is therefore 12.
- All atoms of an element have the same amount of protons, however different numbers of neutrons might exist. As a result, differing mass numbers can exist among atoms of the same element; these are referred to as isotopes.
- An electron carries very little weight. As a result, an atom’s mass number and atomic mass are nearly equal.
Atomic Number
The atomic number of an atom is determined by the total number of protons in its nucleus.
It is symbolized by the letter ‘Z.’
- All atoms of a given element have the same amount of protons and thus the same atomic number.
- Atoms from various elements have distinct atomic numbers.
- For example, all carbon atoms have an atomic number of six, whereas all oxygen atoms have eight protons in their nuclei.
How to Write Nuclear Symbol Notation?
Identify the element: Start by identifying the element using the given atomic number, or the number of protons. For example, calcium (Ca) is an element if there are 20 protons.
Calculate the Mass Number: Add the protons and neutrons together to get the mass number. The mass number is 42 if there are 20 protons and 22 neutrons.
Compose the Nuclear Symbol: Combine the mass number, element symbol, and atomic number to form the nuclear symbol. Thus, 4220Ca, or calcium-42, would be the notation for an atom with 20 protons and 22 neutrons.
Finding the Number of Neutrons: Remember that the mass number (A) denotes the total number of protons and neutrons, whereas the atomic number (Z) shows the number of protons.
- Calculate Neutrons: To find the number of neutrons, subtract the atomic number from the mass. An atom of carbon-14 (146C) contains 6 protons. Subtracting 6 from 14 yields 8 neutrons.
- Using the Periodic Table: Alternatively, when given the mass number and element symbol, use the periodic table to determine the atomic number. Then, subtract the atomic number from the mass number to get the number of neutrons.
Video on Nuclear Symbol Notation
References
- https://www.expii.com/t/isotope-notation-overview-examples-11142
- Helmenstine, Anne Marie, Ph.D. “How to Write the Nuclear Symbol of an Atom.” ThoughtCo, Apr. 5, 2023, thoughtco.com/write-the-nuclear-symbol-of-an-atom-609562.
- https://byjus.com/chemistry/isotopic-notation/
- https://byjus.com/chemistry/atomic-number-mass-number/
- https://sciencenotes.org/nuclear-symbol-notation/

