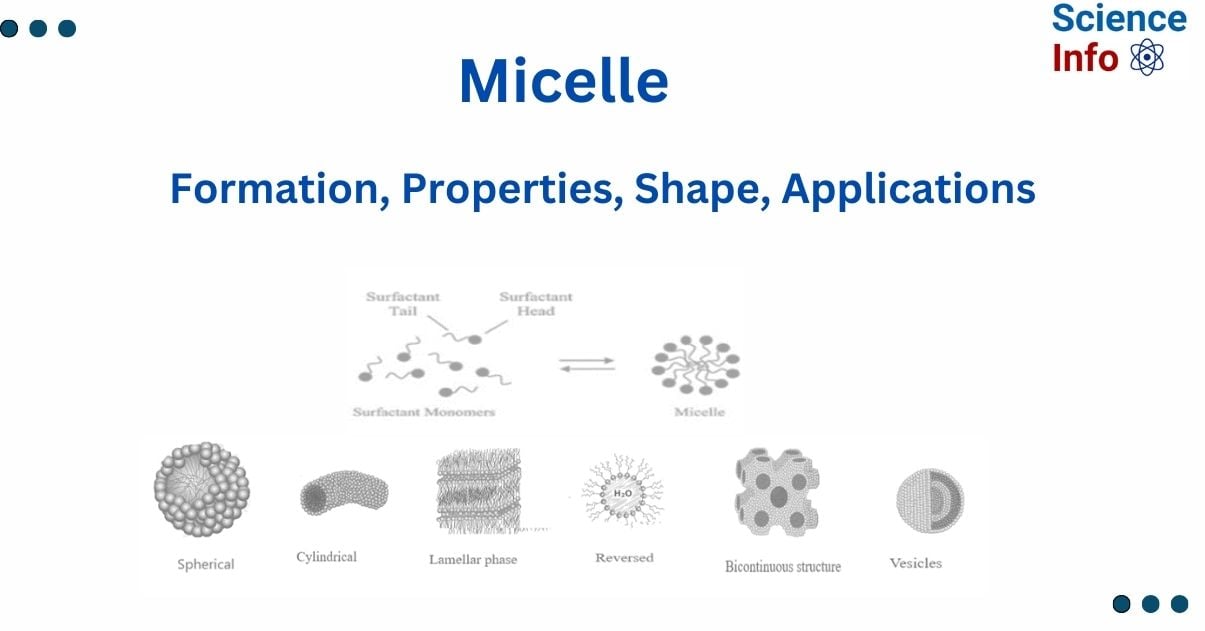
Micelle is an assembly of amphiphilic molecules suspended in a liquid. Surfactants dissociate in water, resulting in a colloidal suspension. They, as defined by IUPAC, are colloidal particles that exist in equilibrium with the molecules or ions in their solutions.
They are highly valuable in the pharmaceutical industry because they can transport medications into liquids, increasing the solubility of some chemicals that are insoluble or only partially soluble. The creation of micelles is determined by the amount and shape of amphiphilic molecules, as well as the temperature and solvent characteristics. Adding amphiphilic molecules to a solvent causes them to self-assemble into micelles when the concentration exceeds a certain level. The system’s differential free energy acts as a driving force for the creation of micelles. This concentration is known as the critical micelle concentration (CMC).
Interesting Science Videos
What is micelle?
Surfactant molecules that exceed the critical micelle concentration (CMC) form micelles, which play a crucial role in science and pharmacy by increasing the dissolution of mildly soluble substances. In a micelle, hydrophobic tails adhere to the inner core to reduce water contact, while hydrophilic heads remain at the outside surface to maximize water interaction. These structures are mobile and frequently form and dissociate in solution.
A micelle is a cluster of amphiphilic molecules scattered in a liquid. Surfactants dissociate in water, resulting in a colloidal suspension. They are also known as associated colloidal systems.
What is Reverse micelle?
It is also known as an inverted micelle. In this micelle, the orientation of the surfactant molecules is reversed from that of a typical micelle. The hydrophilic heads of the surfactant molecules in an inverted micelle face inward towards the center, while the hydrophobic tails point outward towards the non-polar or oil-like surroundings around them. This structure is generally seen in non-aqueous solvents such as oils. The polar (hydrophilic) portions of the molecules avoid the solvent and congregate, resulting in an internal aqueous phase.
Inverted micelles play an essential role in a variety of applications, including protein and enzyme extraction in non-aqueous settings, as well as certain types of nanotechnology and materials research. They form unique shapes and enclose molecules within their water-based core.
Micelle Formation
A micelle is spherically shaped and made up of surfactant molecules with hydrophobic tails concealed from the surrounding liquid by hydrophilic heads. This structure reduces the system’s free energy, resulting in the spontaneous creation of micelles when the concentration of surfactant molecules surpasses a specific limit, which is known as the critical micelle concentration (CMC).
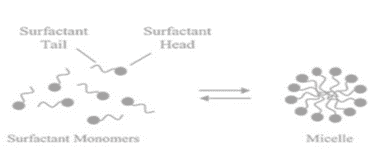
Shape and size of the micelles
They are labile structures formed by the non-covalent combination of individual surfactant monomers. They can be spherical, cylindrical, or planar (discs or bilayers). Their shape and size can be controlled by surfactant chemical structure and solution conditions such as temperature, concentration, composition, ionic strength, and pH.
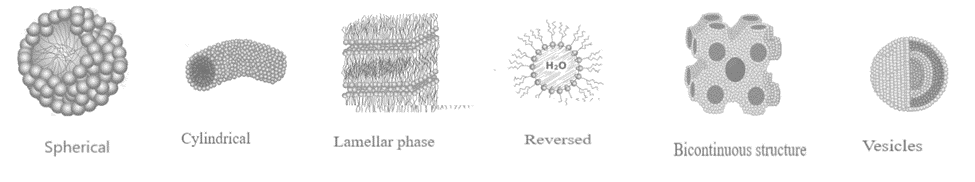
- Spherical micelles: They have an interior made of hydrocarbon chains and a polar head group surface that covers water. The hydrocarbon core has a radius similar to the length of an alkyl chain.
- Cylindrical micelles: They consist of hydrocarbon chains and polar head groups on the aqueous surface. The hydrocarbon core has the same cross-section as spherical micelles. The micelle length fluctuates, indicating polydispersity.
- Lamellar phase micelle: Surfactant bilayers consist of lamellar liquid crystals with a surfactant water system. The hydrocarbon core is 80% the length of two extended alkyl chains.
- Reversed or inverted micelle: The core of a reversed or inverted micelle is water, whereas the polar head contains surfactants. The continuous medium consists of non-polar solvents and alkyl chains. They, like conventional micelles, can form cylindrical structures.
- Bicontinuous structure: A bicontinuous structure with surfactant molecules arranged into linked films.
- Vesicles: They are made of bilayers, similar to the lamellar phase, and have two water compartments: one for forming the core and one for the exterior medium. Vesicles can vary in shape, including reversed types.
Properties of Micelle
- They dissolve hydrophobic compounds in their hydrophobic core, which is critical for their function as detergents.
- Depending on conditions like temperature and surfactant concentration, micelles change their size and shape.
- They are not static. Their constituent molecules continuously exchange with the surrounding solution.
Critical Micelle Concentration
They begin to develop at a specific concentration level. The concentration of amphiphilic molecules is known as the critical micelle concentration (CMC). The critical micelle concentration (CMC) refers to the concentration of surfactants that causes micelles to develop and directs additional surfactants to the micelles. The CMC refers to the precise amount of surfactant required for aggregates to become thermodynamically soluble in a liquid solution.
CMC is an essential surfactant property. Surface tension varies significantly with surfactant concentration before attaining CMC. The CMC of a dispersant in a medium varies according to temperature, pressure, and the presence of surface active chemicals and electrolytes. One can determine the CMC of a surfactant by assessing its physical attributes, including detergency, osmotic pressure (π), surface tension (γ), conductivity (κ) for ionic surfactants, etc.
Factors Affecting Critical Micelle Concentration(CMC)
Factors influencing the (CMC) It is crucial to research the variables that may have an impact on the CMC because the characteristics of the solution change dramatically after micelles form.
1. Amphiphile structure:
- As surfactants become more hydrophobic, their CMC decreases.
- Ionic surfactants often have higher CMC in aqueous solution compared to non-ionic surfactants.
2. Experimental conditions:
- High temperature leads to decreased hydration of hydrophilic groups, promoting micellization (low CMC). High temperatures may destabilize structured water around head groups, increasing CMC.
- pH: CMC will be high when the head group is charged, for example: Low pH for the -COOH head group and high pH for the -NH2 group will raise the CMC in both circumstances.
3. Bulky hydrophobic/hydrophilic groups
- Because it is difficult to incorporate bulky groups into the micelle’s core, surfactant bulkiness raises the CMC.
4. Existence of Additives
- A high ionic strength solution reduces repulsion between head groups due to the presence of counter ions, resulting in a lower CMC.
- Organic chemicals (impurities) may enter micellar areas or alter the interaction between solvent and micelles. Urea and formaldehyde may disrupt the H-bonding network, leading to a rise in CMC.
Polymeric micelles
Polymeric micelles (PMs) are micelles, however, the amphiphilic molecules found in solution are macromolecules. PMs have bigger hydrophobic blocks, resulting in lower CMC values compared to ordinary micelles. Therefore, PMs are more stable in dilute solutions.
PMs are distinguished from traditional micelles by the following properties.
- Larger volume, typically 10–100nm in diameter.
- Lower CMC, however, exists in equilibrium with isolated macromolecules.
- PMs exhibit improved thermodynamic and kinetic stability.
Polymers’ biological stability and diversity make designing hydrophilic and hydrophobic blocks of PMs easier.PMs are typically spherical. Since polymeric micelles are made of amphiphilic block copolymers, they are often more stable than surfactant micelles in physiological fluids. Polymeric micelles’ small size (<100 nm) smoothes the surface, allowing for prolonged retention time in the bloodstream and increased drug concentration at the target region.
Examples
They appear in a range of common substances and products:
- When soap or detergent dissolve in water, the surfactant molecules combine to produce micelles. Oily compounds must be trapped within their hydrophobic cores for cleaning to be effective.
- Many cosmetic cleansers, including micellar water, contain surfactants that create micelles. These remove oil, makeup, and grime from your skin without drying it out.
- In food preparation, certain emulsifying agents (such as lecithin in chocolate) create micelles that stabilize oil-water combinations.
- Bile salts produce micelles in the digestive system, aiding fat absorption. These micelles contain fatty acids and cholesterol, facilitating their passage through the gut lining.
Application of micelle
- Inverse micelles are commonly utilized in food technology to extract proteins and enzymes.
- They are commonly employed as a separating medium in electrophoresis and chromatography.
- They operate as emulsifiers when surfactants exceed the critical micelle concentration, allowing an otherwise insoluble chemical to dissolve. For example, detergents can be used to clean lipophilic compounds that are less soluble and cannot be removed with water alone.
- In drug delivery systems, micelle production improves the solubility of hydrophobic medicines, increasing their absorption and efficacy.
- They are referred to as efficient favorable nano-carriers in a variety of applications, including gene transfer, diagnostic imaging, and medicines.
References
- https://www.sciencedirect.com/topics/medicine-and-dentistry/micelle#:~:text=Micelles%20are%20formed%20by%20self,nonpolar%20region%20forms%20the%20core.
- https://mgcub.ac.in/pdf/material/202004281024188ecdd9fc9d.pdf
- https://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/chemistry/10.physical_chemistry-iii/27.micelle_and_critical_micelle_concentration/et/5516_et_et.pdf.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6071246/
- https://www.aimspress.com/article/doi/10.3934/matersci.2021035?viewType=HTML
