What is Atomic Structure?
The basis for grasping the distinguishing features of matter is the framework of an atom. Protons, neutrons, and electrons are the three foremost subatomic units that make up an atom’s structure. The atom’s nucleus holds the protons and neutrons, whereas the electron’s orbit lies in discrete energy levels. The electron is inversely charged, the neutron is neutral, and the proton is positively charged. The combined presence of protons and neutrons establishes an atom’s mass, notwithstanding the number of protons, or atomic number, that points to the chemical ingredient.
The basic laws of forces, encompassing electromagnetism, the strong nuclear force, and the weak nuclear force, control the interactions between atoms, which are the basic pillars of matter. In nuclear science, where the spotlight moves from the outer electron shell to the nucleus at the atom’s center spot, atomic structure and behavior are significant.
Discovery of the Nucleus: The Alpha-Particle Scattering Experiment
One of the most important turning points in scientific history was the discovery of the atomic nucleus. Ernest and Rutherford conducted the widely recognized alpha-particle diffraction experiment in 1909. A stream of alpha particles, or helium nuclei, was aimed onto a small piece of gold foil by Rutherford, Hans Geiger, and Ernest Marsden. Following the particles’ transit through or deflection by the foil, they examined the scattering forms.
The overwhelming majority of the alpha particles ran straight through the foil with hardly any deformation. The minority of the particles bounced straight towards the source, though, and a tiny percentage were repelled at sharp angles. Rutherford arrived at the conclusion that the atom comes mainly from unoccupied space, but that it also boasts a tiny, dense center that he named the nucleus. The core of the atom incorporates roughly all of the atom’s mass and positive charge. This revelation radically altered our knowledge of the atom by refuting J.J. Thomson’s earlier “plum pudding” theory, which postulated that electrons were ingrained inside a positively charged sphere.
The Nuclear Model of the Atom
The nuclear version of the atom originated after Rutherford’s discovery. The aforementioned idea declares that protons and neutrons build up the atom’s robust, positively charged nucleus, and that electrons orbit it at different energy levels, or shells. Numerous scientific findings, including the stability of atoms and the discrete nature of atomic spectra, have been clarified by this premise.
Later, Niels Bohr enhanced the theory of nuclear structure through the inclusion of the proposal of quantized electron orbits, which explained why electron motion in an atom is consistent. Bohr’s model stipulates that electrons are restricted to precise discontinuous energy levels and that each time they move across these levels, they absorb or emit energy in quantized values. Rutherford’s nuclear model and the subsequent advancements in quantum mechanics were connected by Bohr’s work.
The sturdy nuclear force, one of the four fundamental forces of nature, holds protons and neutrons firmly in order to create the nucleus itself. Given that it functions predominantly inside the nucleus to conquer the repellent electrostatic forces produced by the positively charged protons, the potent nuclear force is a close-range force that is significantly greater than the electromagnetic force.
Nucleon Number vs. Proton Number
The proton number, occasionally referred to as the atomic number and represented by the letter Z, and the nucleon number, also called the mass number and depicted by the letter A, are two imperative numbers that reflect an atom’s characteristics.
- Proton Number (Z): It pertains to the overall amount of protons in the nucleus of an atom. The proton count in an element influences its molecular constitution. Atoms with Z = 1 are all hydrogen atoms, for instance, whereas helium atoms are all Z = 2 atoms, and so forth. The element’s chemical actions and spot on the periodic table are determined by its proton number.
- Nucleon Number (A): It represents the entire amount of protons and neutrons that render up an atom’s nucleus. The proton number (A ≥ Z) is perpetually more than or equal to the nucleon number, which is constantly a whole number. Subsequently neutrons are electrically inactive; they offer an atom bulk but not charge. The total number of participants of the protons and neutrons in the nucleus is approximately comparable to the mass of an atom.
As a consequence, the element can be described by its atomic number (Z), and its distinct isotope is defined by its mass number (A).
Isotopes: Variations of Elements
Atoms of exactly the same element (with the identical amount of protons, or Z) yet distinct amounts of neutrons are called isotopes. In turn, isotopes of identical elements differ for their atomic mass nonetheless share identical chemical features. The three identified isotopes of the hydrogen atom features tritium (1 proton, 2 neutrons), deuterium (1 proton, 1 neutron), along with protium (1 proton, 0 neutrons).
Isotopes tend to be recognized by their mass number preceding their chemical symbol. As a glance: carbon-12 (¹²C) features six protons and six neutrons, whilst carbon-14 (¹⁴C) picks six protons and eight neutrons. Several isotopes hold their shape, whereas others are radioactive and unpredictable, triggering radioactive decay.
The potential uses like nuclear medicine (e.g., the use of radioactive isotopes in imaging and therapy), radiometric dating (e.g., carbon dating using ¹⁴C), and the study of atomic behavior in many scientific domains all heavily rely on a variety of isotopes.
Nuclide Notation: Representing Atoms
Whenever portraying atoms, nuclide terminology is employed to identify the elements and their specific isotopes. Nuclide notation is often displayed as zAX.
Where:
- X is the chemical identity of the element.
- Z pictures out the atomic number (entire number of protons).
- A displays mass number (entire number of nucleons).
For an instance, uranium-235 is represented as: 92 235U
It is evident from this notation that uranium-235 contains 235 nucleons and 92 protons. Atoms and isotopes in radioactive reactions and disintegration processes can be concisely and methodically stated by using nuclide notation.
Conservation Laws in Nuclear Processes
The vigorous chemical reactions and hence the actions of particles throughout the reaction is modulated by a number of conservation principles in nuclear physics. These consist of:
Conservation of Mass-Energy: Under the terms of this law, an enclosed system’s entire mass and energy never alter. Einstein’s prominent equation E = mc2 outlines the way mass can be transformed into energy and vice versa in nuclear reactions. In nuclear fission and fusion, where barely any of mass gets turned into a great amount of energy, is clearly discernible..
Conservation of Momentum: A confined system’s total momentum cannot be altered. The momentum prior to and following a nuclear reaction must be identical.
Conservation of Charge: Nuclear activities sustain the entire electric charge. For a quick glance, in order to maintain the same total charge preceding and following beta decay, the amount of protons and electrons in a nucleus is improved.
Conservation of Nucleon Number: In nuclear processes, the total value of nucleons (protons along with neutrons) remains conserved. In a case of fission, although the individual isotopes may change, the nucleon number before and following the reaction stays constant.
Proper recognition of the behavior of atoms and nuclei in various nuclear processes requires an awareness of these conservation rules.
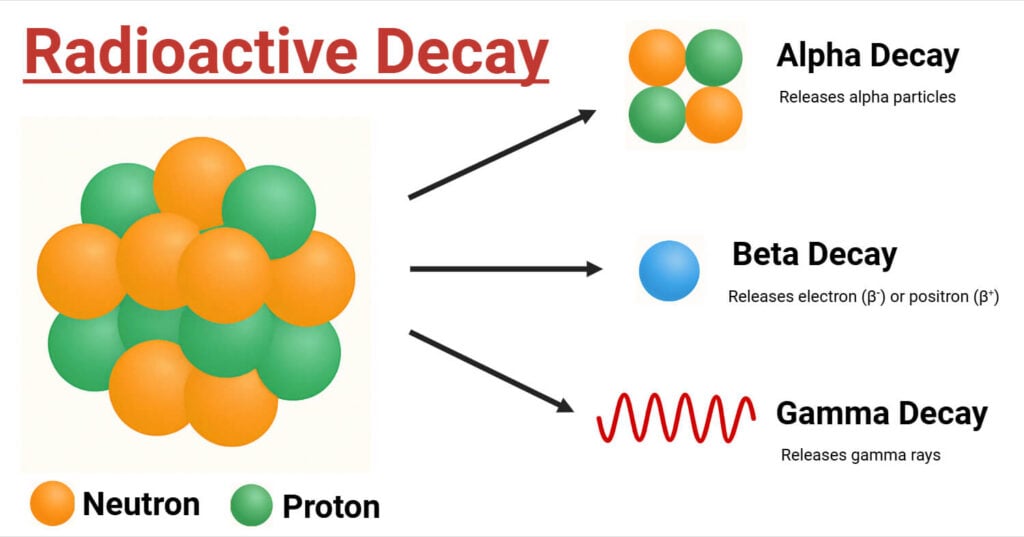
Types of Radiation: Alpha, Beta, and Gamma
The release of energy exactly as electromagnetic waves or particles is known as radiation. Alpha (α), beta (β), and gamma (γ) radiation are the three main kinds of radiations. Every kind of radiation has unique qualities and traits.
Alpha Radiation (α)
Alpha particles, that constitute helium nuclei (two protons and two neutrons), contribute to alpha radiation. These particles exhibit a positive charge and are comparatively hefty. Alpha particles can be obstructed by a sheet of paper or even human skin because of their enormity and low penetrating power. However, because alpha radiation can harm inside tissues, it can be harmful if the radioactive material is swallowed or aspirated.
Beta Radiation (β)
Beta particles, which are highly energetic, fast-paced electrons (β−) or positrons (β+) expelled from the nucleus during an occasion called beta decay, make up beta radiation. Since they are lighter than alpha particles, beta particles possess a greater likelihood of penetrating materials. A thin film of glass or plastic may prevent beta rays. A phenomenon when a proton breaks down into a neutron, yielding a positron and a neutrino, or when a neutron in the nucleus disintegrates into a proton, liberating an electron and an antineutrino, is known as beta decay.
Gamma Radiation (γ)
Photons with tremendous energies are electromagnetic waves that make up gamma radiation. Of the three forms of radiation, gamma rays have the greatest probing strength aiding neither mass nor charge. Extensive shielding, such as numerous centimeters of lead or meters of concrete, is a necessity to avert exposure to gamma radiation since it has the potential to permeate deep layers of either material. Once the nucleus changes to a lower energy state, gamma radiation is frequently distributed in addition to alpha or beta decay.
Antiparticles and the Positron
The contrary of a particle in particle physics i.e. an antiparticle bears the reverse charge alongside other quantum features. As a quick sneak, positron is the electron’s antiparticle. The positron possesses equivalent mass as the electron however has a positive charge instead. Gamma radiation arises when an electron and a positron encounter and annihilate themselves. A pivotal concept in the investigation of antimatter is this mechanism, which is known as electron-positron annihilation.
Neutrinos and Antineutrinos in Beta Decay
Within the nucleus, a neutron experiences beta decay when it converts into a proton or the other way around. Neutrinos and antineutrinos escape in addition to the electron or positron. Neutrinos are neutral, exceptionally thin particles that are capable of weak interaction with matter. Wolfgang Pauli hypothesized them in 1930 as a way to articulate why beta decay reactions were lacking energy.
Neutrinos appear in two varieties: muon neutrinos (νμ) and electron neutrinos (ve). For the purpose of safeguarding the reaction’s equilibrium in energy, an electron antineutrino (νˉe) is released together with the liberated electron during beta decay.
Energy Distribution in Alpha and Beta Decay
In alpha and beta decay processes, the energy discharged is not necessarily dispersed equally. The energy generated amid alpha decay tends to be divided throughout the alpha particle and the remaining nucleus. The binding energy of the nucleus and the energy dissipated during decay govern how much energy flows to the alpha particle. The daughter nucleus, the neutrino, and the expelled electron all receive the energy during beta decay.
Since it can reveal information about the inner workings and binding energies of the decaying nucleus, this energy distribution has significance to grasp nuclear processes.
Writing and Balancing Nuclear Equations
Nuclear reactions and disintegration events are symbolized by nuclear equations. Both the overall charge and the aggregate amount of nucleons must be preserved in these equations. In one instance, the release from an alpha particle in alpha decay is displayed in the following manner:
ZAX→ z-2A-4Y+ 2 4α
Having the nucleon number and charge equivalent on both sides, this equation depicts how the parent nucleus (X) disintegrates into a daughter nucleus (Y) and an alpha particle.
An electron and an antineutrino are given away when a neutron in the nucleus breaks down into a proton in beta decay:
N → p+β−+νˉe
It counts to ensure that the atomic number and total mass number are in harmony for all kinds of disintegration.
The Unified Atomic Mass Unit (u)
A universal standardized measurement of mass in atomic and nuclear physics is the unified atomic mass unit (u). It has the mass of one twelfth of an atom of carbon-12 and one atomic mass unit is analogous to 1.66 × 10⁻²⁷ kg. The masses of atoms, isotopes, and subatomic particles are put forward in an improved comprehensible fashion via the unified atomic mass unit.
Conclusions
Our awareness of all existing matters and the cosmos relies on the investigation of atoms, nuclei, and radiation. The scientific knowledge of the atom was altered by the advancement of the field of atomic theory and the recognition of the nucleus. Throughout nuclear energy generation to medical imaging and therapy, a sense of isotopes, nuclear decay, and radiation enables a wide range of beneficial applications. Nuclear physics serves as an essential branch to the contemporary globe owing to the way fundamental forces interact and how conservation rules are used in nuclear procedures, which propel scientific and technological breakthroughs.
References
Turner, J. E. (2008). Atoms, radiation, and radiation protection. John Wiley & Sons.
Vallabhajosula, S. (2023). Atoms and radiation. In Molecular Imaging and Targeted Therapy: Radiopharmaceuticals and Clinical Applications (pp. 37-47). Cham: Springer International Publishing.
Gray, L. H., & Tarrant, G. T. P. (1932). The nature of the interaction between gamma-radiation and the atomic nucleus. Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character, 136(830), 662-691.
Ishkhanov, B. S. (2012). The atomic nucleus. Moscow University Physics Bulletin, 67(1), 1-24.
Charlesby, A. (2016). Atomic radiation and polymers: international series of monographs on radiation effects in materials. Elsevier.
Povh, B., Rith, K., Scholz, C., Zetsche, F., & Rodejohann, W. (1995). Particles and nuclei. Berlin, Heidelberg, NY: Springer-Verlag.
