Pressure is defined as an unseen force, present across a surface, showing “by what force something pushes inside a certain space”. We interact with pressure all around us. The very common example- the air we inhale or exhale exhibit pressure. Other mechanical systems, atmosphere and everything are affected by pressure.
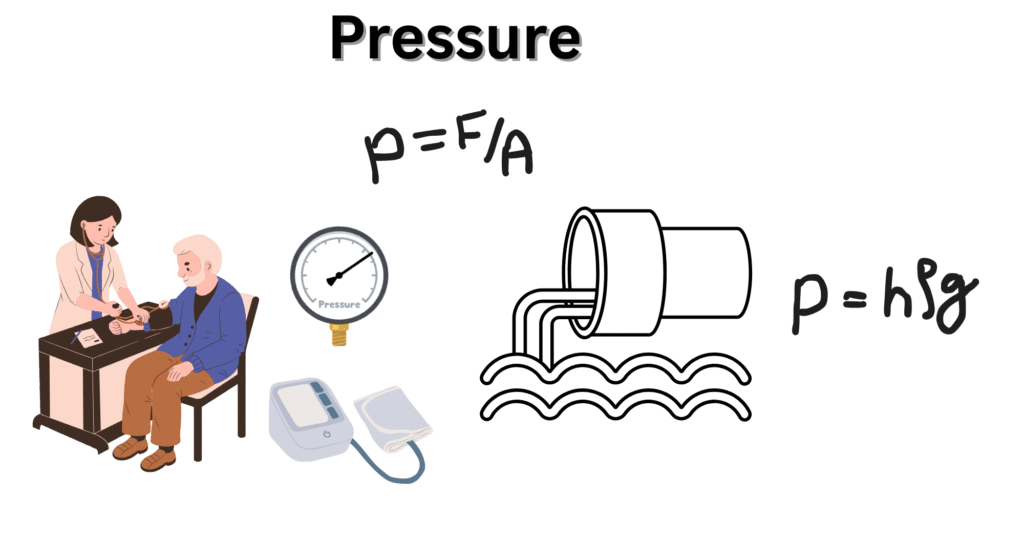
Definition of Pressure and the Basic Formula
Force can be applied perpendicularly on a surface area which generates pressure. Mathematically, it is expressed as:
Pressure = Force /Area [Equation 1]
The SI unit of pressure is Newton/(meter)^2 or pascal (pa). Similarly, the CGS unit is barye (Ba) (1 Barye = 1 dyne·cm^(−2) or 0.1 Pa).
We can see that force and pressure are directly proportional but pressure is inversely related with the surface area.
Also, in fluid dynamics, liquid pressure is related to depth of fluid (h), density of fluid (⍴) and the acceleration due to gravity (g) as.
OR, Pressure (P) = h⍴g [Equation2]
SI Unit Pascal and Other Common Pressure Units
The pascal unit is named so after the French physicist Blaise Pascal who invented the law and expression for pressure.Generally kilopascals (kPa) or megapascals (MPa) are preferred for calculations. Some other units are:
- Atmospheric pressure: It is the standard reference for pressure, defined as the force present on the air molecules by earth at a specific location and time. (1 atm = 101.325 pa). The sea level is the standpoint for the measure of atmospheric pressure.
- Bar: (1 bar = 100,000 Pa OR 100 kPa) is an extremely used term in meteorology and not much in other realms..
- Millimeter of mercury (mm of Hg): This unit is taken by raising Mercury pressure in a column generally by 1 millimeter..
- Torr: It is only used in research based studies, certainly in high-vacuum physics and engineering (1 Torr = 1/760 atm).
All these units are valued accordingly in their specific fields.
Types of Pressure
Pressure can be assumed of several forms based on its nature and the way force is applied. All the types are equally important to deal with the physical phenomena around us.
Atmospheric Pressure
The air resistance on earth applies knowing or unknowing forces to everything present in it. It may essentially affect the weather, breathing or a flight. Sea level has the greatest atmospheric pressure which continues to fall with the altitude.
Gauge Pressure
Gauge pressure keeps atmospheric pressure as the base. It excludes air pressure variations and only measures pressures that are higher or lower than ambient. Gauge pressure takes measurement in relation to the atmospheric pressure and gives a more precise measurement of the pressure exerted by a fluid or gas in a closed system.
Absolute Pressure
Absolute pressure has a direct association with both atmospheric and additional pressure. This type is developed to get a precise measurement of pressure for scientific and industrial purposes.
Pabs=Patm+Pgauge
Differential Pressure
The term simply indicates difference. It compares the pressure between two points and notes the difference. It is most useful in engineering and fluid systems.
Key Factors That Influence Pressure
Various factors affect pressure which are given below:
- Force Applied: As seen from equation [1], P∝F. Thus, greater force has a positive impact on pressure and negative impact on its reduction.
- Area: Also from equation [1], P∝1/A. Thus a larger surface area employs less pressure and vice-versa.
- Fluid Density: From equation [2] we see P∝⍴. Hence density shows a positive impact on pressure.
- Depth of fluid: P∝h from [1]. Thus, depth also has a positive impact on the fluid pressure.
Pressure in Fluids: Liquids vs. Gases Explained
All flowing matters are the fluids (liquids and gases) and bear pressure but behave according to their nature, uniquely as per the properties.
In liquids, pressure acts on the basis of their depth and density. Their molecules are densely arranged and don’t change much in volume. Thus greater pressure is realized when we go deeper underwater. One can take any direction, but the depth remains the same and pressure rises in the same way as told by equation [2].
Gases can flow everywhere, being easily compressible. Thus, its pressure behaves differently. It depends on the volume and temperature of the surrounding area and also the number of gas particles present in a particular place. These gas particles cannot stay at one place with no movement so, keep on hitting each other in addition to the walls of the vessel.
In brief, liquid pressure is steadier than that of gas pressure as the effect of temperature is rigorous on gases. In fluid dynamics, the proper knowledge of these differences is very useful. (Also read about Floatation).
Pascal’s Principle and Hydraulic System Applications
Pascal’s Principle is developed to describe the pressure of a liquid enclosed to something. He argues that, if pressure at any point inside gets disturbed then it supplies as it is without any loss to all portions. All hydraulic systems follow this principle for operations. Any liquids or semi-liquids, tightly confined in a container shows this effect which is in accordance with Pascal’s law.
This law has turned very beneficial to the applications like, braking systems and hydraulic lifts where intentional pressure exerted at one end supplies pressure to the other end for generating required action. It is also equally reliable in high altitudes which controls aircrafts.
Devices for Measuring Pressure
The devices used for measuring pressure are as follows:
Manometers:
They are used to measure the pressure of fluids which further include three types Types include:
*U-tube manometer: As indicated by the name, it is a U-shaped container half filled with liquids like water or mercury. It is a differential method of measuring pressure which compares the pressure of the same liquid at two ends (one in contact with a gas and other left free).
*Inclined manometer: It is a slight modification of the U-tube manometer and contains a tilted tube also half filled with liquids.It can detect the small pressure fluctuations and give precise reading.
Barometers:
Barometers are used to measure the atmospheric pressure at altitudes.
Pressure Sensors:
Modern pressure sensors convert pressure into an electrical signal which allows digital readings. They are integrated into automated systems. They make the pressure reading process easier by providing digital information.
Barometric Pressure and Its Role in Weather Forecasting
Barometer is the tool for measuring atmospheric pressure, showing the weather fluctuations and predicting what is next. The pressure fluctuations in wind, cloud and precipitation is measured by the barometers and the information like weather changes, storms, clouds, rainfall etc. are forecasted by the meteorologists. The coming up weather condition is also determined with the measurement of pressure conditions of above factors.
Blood Pressure: What It Means for Human Health
Blood also applies a force to pass from a blood vessel which is called the blood pressure. Sphygmomanometer is used for tracking human blood pressure that expresses the result in mm of Hg. Blood pressure is a serious issue as the patients with high blood pressure are increasing day by day. High blood pressure is the high pressure generated by blood against the blood vessels causing damage to them or also a heart attack. On the other hand low blood pressure causes loss of haemoglobin, iron and oxygen. Thus blood pressure must be kept balanced. 120/80 mm of Hg is the marked systolic and diastolic blood pressure of a grown up man.
Everyday Uses of Pressure in Tools and Technology
Pressure tracking is very essential for many everyday tools and technologies. In car tires, the pressure gauges used to check the tire pressure air which ensures smooth travel. Hydraulic systems employ pressure checking on everything like brakes, lifting, heavy machinery, excavators etc. to carry everything easily. Similarly pressurized air is applied in drilling and painting by pneumatic tools. Pressure cookers generate the pressure of steam inside the pot after the lead is shut, which makes cooking faster. Aerosols apply pressure to the fluids to come out evenly. Blood pressure monitors and ventilators are major important devices in the hospitals to safeguard a patient’s health condition.
Negative Pressure and Vacuum Concepts Made Simple
The negative pressure can be achieved by sucking the air lying inside an enclosed area and creating a vacuum. This helps in food packaging which removes air or bacteria that damages the food or grocery while sealing and keeps the item fresh for the long term. Suction pumps like vacuum cleaners also apply negative pressure to create a pressure difference and perform their tasks.
Pressure-Temperature Links in the Gas Laws
Gay-Lucas beautifully illustrated the nature of pressure according to the temperature and stated P∝T, keeping the volume as it is.
Ideal gas equation shows a definite relation of pressure, volume, temperature and the number of gas particles as,
PV=nRT [Equation 3]
Here,
- P = Pressure
- V = Volume
- n = Number of moles
- R = Ideal gas constant
- T = Temperature in Kelvin
Safety Concerns When Working with High Pressure
Dragging yourself to a high pressure zone may be vulnerable. High-pressure operation devices can cause a tear, burn, leakage or injury if mishandled. It can also cause internal damage like insertion of fluid to the body, cuts or flying debris damage etc. Therefore a proper working guide must be regulated. Precaution measures are to be taken carefully before handling such tasks. Danger or restriction symbol, red lights must be assigned to the areas exposing high pressure systems. Safety measures are the major thoughts to be employed concerning high pressure.
Pressure Unit Conversions
Some common pressure conversions are listed below:
- 1 atm = 101,325 Pa
- 1 bar = 100,000 Pa
- 1 psi = 6894.76 Pa
- 1 torr = 1/760 atm
Conclusion
Pressure is that invisible force which guides various aspects of our daily lives like the heart pulse to cooking and even advanced works like machine operations. It has a powerful impact on diverse realms of physics, engineering, meteorology, medicine etc. Thus we can call pressure not only a scientific term, but a reason for survival and great innovations. The proper governance of pressure in fluids or solids helps in performing our daily tasks smoothly. Pressure maintenance also drives technologies like hydraulic lifts, weather forecasting and also home appliances through positive or negative pressures.
Pressure also teaches us about balance. A slight lag in balance can harm a machinery to a blood stream. As science and technology is advancing day by day, the principles of pressure will keep on driving it towards eternity. By acknowledging the practical and theoretical values of pressure, we not only sharpen our scientific understanding but also involve ourselves to navigate a world governed by forces.
References
Pressure being a scalar quantity
https://www.britannica.com/science/fluid-mechanics/Turbulence
https://byjus.com/physics/pressure/
https://www.sciencing.com/pressure-physics-definition-units-formula-examples-13723383/
What Is Pressure in Physics? Understanding Force per Area

Well explained