Radioactive decay is an integral concept in nuclear physics and chemistry that describes how unstable atomic nuclei lose their energy by producing radiation. This natural phenomenon is not only important for understanding the behavior of matter at the atomic level, but it also has extensive implications in a variety of scientific, industrial, and medical fields. In this article, we will dig into the complexities of radioactive decay, including its unpredictability, mathematical basis, and practical applications.
What is Radioactive Decay?
Atoms are made up of protons, neutrons, and electrons, with the nucleus comprising protons and neutrons held together by nuclear forces. In some isotopes, these forces are inadequate to preserve stability, resulting in the release of energy or particles in an effort to establish a more stable condition. We know this mechanism as radioactive decay.
Nuclear substances emit radiation in many forms, such as alpha particles, beta particles, and gamma rays. Emissions like those may modify the ratio of protons and neutrons in the nucleus, switching one element into another.
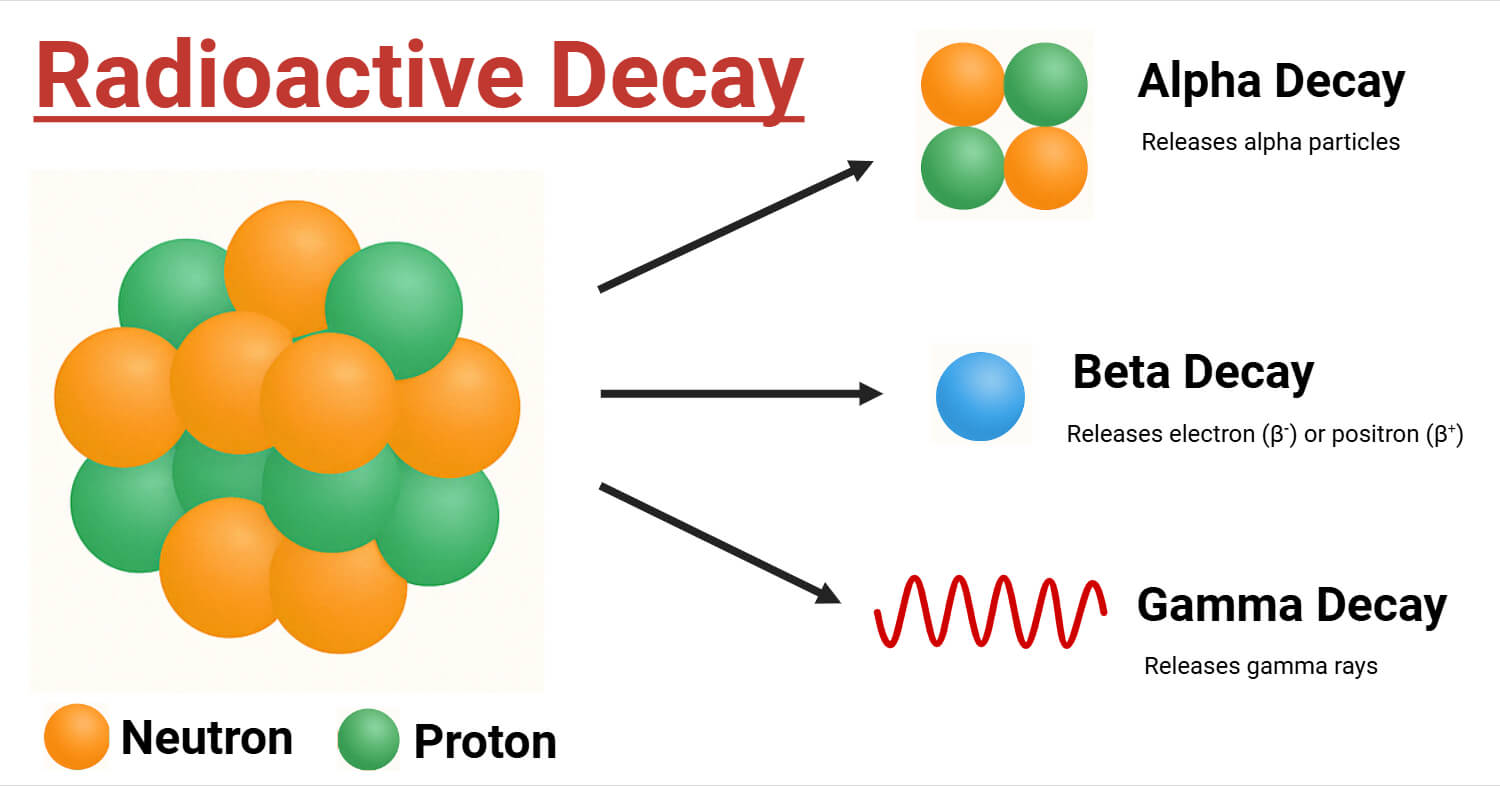
Types of Radioactive Decay
Understanding the mechanics and implications of radioactive decay is critical for a variety of professions, including health, archeology, and paleontology. Radioactive decay takes several forms, depending on how an unstable nucleus attempts stability. The primary types are:
Alpha Decay
In this type, the nucleus emits an alpha particle, which is made up of two protons and two neutrons. Here the atomic number reduces by 2 and the mass number by 4. As a prime instance, Uranium-238 breaks down into Thorium-234 and emits an alpha particle.
Beta Decay
A neutron in the nucleus is converted into a proton, emitting an electron and an antineutrino, or vice versa, where positron and neutrino are emitted. For both scenarios, the atomic number alters by one, but the mass number stays constant.
Gamma Decay
The entire procedure involves the discharge of electromagnetic radiation from the nucleus. It typically happens after alpha or beta decay, as the nucleus transitions from a higher energy state to a lower one. The overall number of protons and neutrons in the nucleus remains unaffected.
Electron Capture
During electron capture, the nucleus traps an orbiting electron, converting a proton into a neutron. This phenomenon drops the atomic number by one without influencing the mass number.
Spontaneous Fission
This type is common in very heavy elements like uranium-235 or plutonium-239. The nucleus breaks down creating two or more smaller components, releasing more neutrons and energy.
The Random Nature of Radioactive Decay
Radioactive decay can be practically unpredictable, making it difficult to anticipate when a particular nucleus will decay. Probability, on the other hand, allows us to statistically describe the behavior of a huge number of nuclei.
Every single nucleus of a radioactive isotope has a predetermined chance of decaying within a specific time period, which is specified by its decay constant. This unpredictability results from quantum physics, which regulates particle behavior at the atomic and subatomic levels. Regardless of the randomness of individual decay incidents, the overall decay rate of the data set reflects a well-defined exponential distribution.
Experiments using Geiger counters and scintillation detectors reveal that decay events occur irregularly and without an apparent pattern. When plotted over time, the number of decay events varies, but the average decay rate follows the decay constant.
The randomized changes in the count rate not only demonstrate the chaotic character of radioactive decay but also justify the probabilistic paradigm of quantum mechanics. Recognizing these movements is critical in areas such as nuclear physics, medical imaging, and environmental radiation tracking.
Spontaneity and Randomness in Decay Processes
Radioactive decay occurs spontaneously, with no outside effect or initiating mechanism. This spontaneity results from the inbuilt volatility of some isotopes. Spontaneous decay is the mechanism by which an unstable atomic nucleus changes into a state of greater stability without any sort of outside input.This alteration is triggered by the nucleus’ intrinsic instability, resulting in the emission of particles or electromagnetic radiation.
Spontaneous decay happens at random and is determined by the nucleus’ half-life, which is unique to the isotope. Radioactive decay is regulated by quantum tunneling, a probabilistic phenomenon in which particles pass through energy barriers. The odds of decay are consistent regardless of isotope but fluctuate across them.
The spontaneity guarantees that decay happens spontaneously and without external influence, whereas randomization ensures that the time of decay is unpredictable for individual nuclei. However, in vast numbers, these random processes produce statistically predictable patterns, making radioactive decay both a random and dependable natural clock.
In contrast, induced decay happens when an external signal causes an unstable nucleus to undergo a transition. This external influence might include high-energy particles, photons, or interactions with other nuclei. Compared to spontaneous decay, induced decay necessitates an intentional addition of energy or interaction.
Probabilistic models are the best way to explain the random nature of decay. As an illustration, given an ensemble of identical radioactive nuclei, each of which has the same chance of decaying during a particular time frame, regardless of other factors like temperature or pressure, this behavior emphasizes the underlying principles of quantum mechanics that regulate radioactive reactions.
Activity and Decay Constant Explained
The radiation activity of a radioactive material can be understood as the percentage of disintegrates per unit time and usually quantified in becquerels (Bq), with 1 Bq being one decay per second.
Activity is determined by the quantity of radioactive nuclei available as well as the isotope’s decay constant.
The decay constant (λ) represents the predicted rate of decay per unit time for each nucleus. The equation,
A = λN (Equation 1)
explains the correlation between activity A and the quantity of undecayed nuclei.
This connection demonstrates that as the number of nuclei drops over time due to decay, so does their activity.
Half-Life: Measuring Decay Over Time
The half-life (T1/2) is a critically important idea in radioactive decay which measures the time required for the fifty percent of the radioactive nuclei in a sample to decay. It is a realistic metric of decay rate that is distinct to each radioactive isotope.
The connection between half-life and the decay constant is:
T1/2 = ln2/λ (Equation 2)
The equation shows that isotopes with high decay constants have shorter half-lives, whereas those with low decay constants decay gradually over time.
Mathematical Relationships in Radioactive Decay
The decay equation outlines the exponential law that regulates the radioactive nuclei’s disintegration.
N(t)=N0e − λt (Equation 3)
Here N(t) denotes the number of undecayed nuclei at time. N0 is the original number of nuclei, λ is the decay constant, and e is the logarithmic scale.
From this equation, we may deduce additional crucial numbers, such as the activity A(t), which likewise falls exponentially with time:
A(t)=A0e − λt (Equation 4)
These mathematical equations allow scientists to anticipate the behavior of radioactive compounds over time with amazing accuracy.
The Exponential Nature of Radioactive Decay
Exponential decay is the primary feature of radioactive processes. It reveals that the pace of decay correlates to the number of undecayed nuclei present. This feature causes a slow but constant decline in activity over time.
Let’s say that after one half-life; just half of the initial nuclei remain. Subsequent to two half-lives, just a quarter remains, and so on. This regularity is critical for applications such as radiocarbon dating, in which the residual fraction of carbon-14 in an old artifact determines its age.
Graphical Representation of Radioactive Decay
Radioactive decay is often shown graphically by charting the number of undecayed nuclei N(t) or activity A(t) with time. These plots provide exponential decay contours, which show how the amount decreases over time.
A logarithmic scale may be employed as well to linearize the decay curve, rendering it simple to interpret data and calculate the decay constant or half-life using experimental results. These visualizations are essential tools for scientists and engineers that deal with radioactive materials.

Applications of Radioactive Decay
Radioactive decay serves multiple uses in broad fields:
- Medicine:
Radioactive isotopes such as technetium-99m are used for diagnostic screening in medical imaging and therapy, while iodine-131 and cobalt-60 are utilized to treat cancer.
- Archaeology:
The technique of radiocarbon dating predicts the age of organic things via monitoring the decay of carbon-14. This method has transformed our knowledge of historical chronology.
- Energy Production:
Nuclear reactors use the energy generated during the decay and fission of radioactive isotopes such as uranium-235 and plutonium-239 to produce electricity.
- Industry:
Radioactive isotopes are used in industrial radiography to check the durability of materials and in tracker investigations to track chemical reactions. Beta radiation contributes to the uniform thickness of materials such as paper, plastic, and metal sheets.
- Radioactive decay research helps scientists comprehend fundamental forces and particles in the cosmos.
Safety Measures in Handling Radioactive Materials
In spite of its beneficial effects, radioactive decay may carry serious health and ecological risks if not handled appropriately. Strict safety precautions must be used while handling radioactive materials to safeguard both the environment and human health.The following safety precautions are needed.
Ionizing radiation, such as alpha, beta, gamma, and neutron radiation, released during radioactive decay, is the leading cause of radiation dangers. These have the potential to harm cells, resulting in either immediate syndrome or chronic health consequences like cancer. In order to evaluate possible dangers, it is essential to understand the kind and energy of radiation.
- Protective Measures
- Relevant materials, such as lead or concrete, should be utilized to protect against radiation.
- Limit the prolonged exposure and increase distancing from radiation sources.
- To avoid contact, wear gloves, safeguards, and respiratory equipment.
- Establish routine radiation assessment and give extensive instructions to staff members before handling radioactive materials.
- Environmental Impacts and Waste Management
If radioactive materials are handled inappropriately, the surrounding may get contaminated. Key components of efficient waste management are as follows:
- Applying safe disposal methods into practice, including deep geological storage for hazardous waste.
- Retrieving usable components from spent fuel through recycling. While long-term solutions concentrate on minimizing waste and its effects, inspection and restriction attempt to prevent environmental damage.
- To identify and reduce pollution, radiation levels in the vicinity should be continuously investigated.
With these preventative measures, radioactive decay hazards can be successfully controlled, protecting both the public and the environment.
Conclusions
A basic procedure that happens to unstable atomic nuclei is radioactive decay, which releases energy as radiation when the nucleus changes into a more stable state. Numerous disciplines, like environmental research, energy production, medical science, and excavations, depend on this natural occurrence.
Understanding the properties of nuclear forces and the enduring nature of matter is possible through the study of radioactive decay. Its importance in expanding human knowledge and enhancing quality of life is demonstrated by applications like radiometric dating, nuclear power generation, and radiotherapy for cancer treatment.
Still, strict safety precautions, laws, and efficient waste management are required to mitigate the risks of radioactive decay, which include radiation exposure and environmental contamination. The creation of technologies and procedures that optimize advantages while lowering dangers is made possible by an awareness of the fundamentals of decay, its types, and its impacts.
To wrap it up, radioactive decay is an important natural phenomenon as well as a useful instrument for research and business. Its multiple fields depend on its careful usage and management to guarantee sustainability and safety.
References
Pfützner, M., Karny, M., Grigorenko, L. V., & Riisager, K. (2012). Radioactive decays at limits of nuclear stability. Reviews of modern physics, 84(2), 567-619.
Lawson, R. S. (1999). An introduction to radioactivity. Nuclear Medicine Department Manchester Royal Infirmary.
Huestis, S. P. (2002). Understanding the origin and meaning of the radioactive decay equation. Journal of Geoscience Education, 50(5), 524-527.
I. Ahmad, J.P. Greene, E.F. Moore, S. Ghelberg, A. Ofan, M. Paul, W. Kutschera, Improved measurement of the Ti44 half-life from a 14-year long study. Phys. Rev. C 74(6), 065803 (2006). https://doi.org/10.1103/PhysRevC.74.065803
D. Arnett, Supernovae and Nucleosynthesis. An Investigation of the History of Matter, from the Big Bang to the Present. Princeton Series in Astrophysics (Princeton University Press, New Jersey, 1996)
Radioactivity: Definition, Types, Formula, and Applications
https://byjus.com/physics/radioactive-decay/
