Interesting Science Videos
Aldehyde Definition
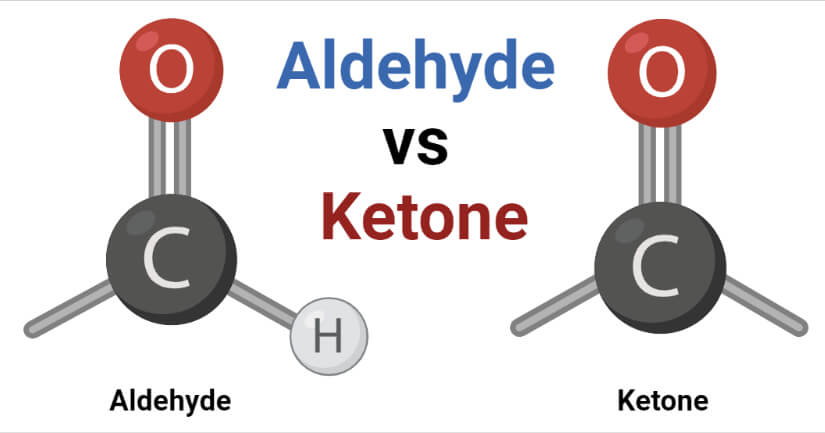
An aldehyde is a type of organic compound containing the functional group with the structure –CHO, where the carbon double-bonded to oxygen is termed the carbonyl group.
- Aldehydes are generally formed by the removal of a hydrogen atom from an alcohol compound.
- The chemical formula of aldehyde is R-CHO where the carbon atom is double-bonded to oxygen and single-bonded to hydrogen and R or an alkyl group. The alkyl group can either be saturated, unsaturated, alicyclic, aromatic, or heterocyclic.
- The –CHO group is also called the aldehyde group, and it occurs at the end of the carbon chain.
- Aldehydes are reactive compounds that can be reduced to form alcohols or oxidized to form carboxylic acids.
- Most of the aldehydes have a pleasant odor and are generally derived from alcohols as a result of dehydrogenation.
- The aldehydes are named with the suffix –al based on the IUPAC system, and no numbering is required as the aldehyde group always occurs at the end of the chain.
- The aldehyde group in aldehyde is polar due to the presence of hydrogen bond, which influences the physical properties of the compound.
- There are various tests are reactions in analytical chemistry that can be used to distinguish aldehyde from other organic compounds.
- These tests are often based on the detection of the aldehyde group as a part of the identification tests of different compounds
- Tests like Fehling’s test and Tollen’s test are performed to differentiate between aldehydes and ketones in a chemical laboratory.
- Aldehydes are essential building blocks in organic chemistry that can be used to synthesize a group of other compounds.
- Aldehydes are of industrial significance as they are mainly used as solvents, perfumes, and flavoring agents. Some are even used in dyes, plastics, and pharmaceutical products.
Ketone Definition
Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (CO) as a functional group.
- The carbonyl group consists of a carbon atom double-bonded with oxygen whereas the remaining two bonds are to other carbon atoms of alkyl groups.
- The alkyl group bound to the carbonyl group can be aliphatic, aromatic, or alicyclic depending on the compound.
- The carbonyl group of ketone doesn’t occur at the end of the chain as it connects two alkyl groups.
- Ketones can be further classified into groups on the basis of their substituents as well as the equivalency of the substituents.
- Ketones can be symmetrical and asymmetrical depending on the nature of the alkyl groups bonded to the carbonyl group. Diketones are compounds with two carbonyl groups.
- Ketones are produced from secondary alcohols via reduction and can be further oxidized to form carboxylic acids.
- According to the naming rules of IUPAC, ketones are named by changing the suffix –ane to –anone. It is important to denote the position of the carbonyl group in the compound.
- There are various tests are reactions in analytical chemistry that can be used to distinguish ketone from other organic compounds.
- These tests detect the carbonyl group present in the compound as a part of the identification tests of different compounds
- Ketones are structurally similar to aldehydes; thus, analytical tests are performed to differentiate between the two.
- Tests like Fehling’s test and Tollen’s test are performed to differentiate between aldehydes and ketones in the chemical laboratory.
- Ketone compounds have important properties as they are found in different sugars and other compounds that are used for medicinal use as steroid hormones.
- Most of the ketones are found in nature, and only a small number of ketones can be manufactured in industries.
- Ketones can, however, be used as intermediates of building blocks of complex organic compounds.
14 Major Differences (Aldehyde vs Ketone)
| Characteristics | Aldehyde | Ketone |
| Definition | An aldehyde is a type of organic compound containing the functional group with the structure –CHO, where the carbon double-bonded to oxygen is termed the carbonyl group. | Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (CO) as a functional group. |
| Functional group | The function group in aldehydes is –CHO. | The functional group in ketones is –CO. |
| Position of the functional group | The functional group always occurs at the terminus of the compound. | The functional group always occurs within the compound and not at the terminus. |
| Substituents | The functional group is bonded to a single alkyl group. | The functional group is bonded to two alkyl groups. |
| Nomenclature | Aldehydes are named by using the suffix – al. | Ketones are named by using the suffix – one. |
| Reactivity | Aldehydes are more reactive than ketones. | Ketones are less reactive than aldehydes. |
| Classification | Aldehydes cannot be classified into subgroups. | Ketones can be divided into groups depending on the number of functional groups and the nature of the alkyl groups. |
| Synthesis | Aldehydes are produced from primary alcohol via reduction. | Ketones are produced from secondary alcohol via reduction. |
| Occurrence | Aldehydes mostly occur in aromatic or volatile compounds. | Ketones can be found in sugars. |
| Oxidation | The oxidation of aldehydes forms carboxylic acid without breaking the carbon chain. | The oxidation of ketones cannot form carboxylic acid without breaking the carbon chain. |
| Fehling’s Test | Aldehydes produce a red precipitate indicating a positive reaction. | Ketones do not produce red precipitation. |
| Tollen’s Test | Aldehydes produce a shiny silver mirror in Tollen’s test. | No silver mirror is produced by ketone compounds. |
| Sodium Nitroprusside Test | Aldehydes do not produce red coloration of the solution. | Ketones produce a red coloration in the solution. |
| Examples | Examples of aldehydes include formaldehyde, acetaldehyde, etc. | Examples of ketones include acetone, butanone, etc. |
Examples of aldehydes
Formaldehyde
- Formaldehyde is the simplest aldehyde compound where the aldehyde group is bonded to hydrogen.
- Formaldehyde is synthesized by the oxidation of methanol and is used as an antiseptic, disinfectant, and general-purpose chemical reagent in laboratories.
- It is a colorless poisonous gas that might result in unconsciousness among other issues under prolonged inhalation.
- The compound instantly polymerizes into paraformaldehyde and thus is often stored in an aqueous form as formalin.
- Formaldehyde is an important chemical substance that is a precursor for many organic compounds.
- Formaldehyde can be found in the natural environment in different sources like the upper atmosphere, within living organisms, and in space.
- In industries, formaldehyde is used for the production of urea resin, polymethylene plastics, and 1,4- butanediol.
- Formaldehyde-based compounds are also used in the manufacture of automobiles as well as components in transmission and electrical systems.
Examples of ketones
Acetone
- Acetone is the simplest aliphatic ketone which is a common organic solvent of industrial as well as chemical importance.
- It consists of a single carbonyl group that is bonded to two alkyl groups (CH3) to form a symmetrical ketone.
- It is a colorless, aromatic, volatile, and viscous liquid that occurs naturally in plants, trees, vehicle exhausts, fire, and fat metabolism.
- Acetone can also be found in low quantities in urine and blood in healthy individuals, whereas the quantity might be higher in diabetic individuals.
- Acetone is widely used in industries due to its ability to dissolve many fats, resins, cellulose, ethers, and esters.
- Besides, it is also used as a chemical intermediate in pharmaceuticals and solvents for cosmetics and paints.
- It is, however, toxic to living beings in high concentration, and different human activities like fire, sewage, and urbanization increase the concentration of acetone in the environment.
References and Sources
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 712, Formaldehyde” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Formaldehyde. Accessed 16 February, 2021.
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 180, Acetone” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Acetone. Accessed 16 February, 2021.
- 3% – https://www.britannica.com/science/ketone
- 1% – https://www.engineeringtoolbox.com/acetone-2-propanone-dimethyl-ketone-properties-d_2036.html
- 1% – https://www.coursehero.com/file/p65of5r/quantities-in-urine-and-blood-larger-amounts-may-be-found-in-the-urine-and/
- 1% – https://www.chemguide.co.uk/organicprops/carbonyls/oxidation.html
- 1% – https://en.wikiquote.org/wiki/Aldehyde
- 1% – https://en.wikipedia.org/wiki/Organic_chemical_nomenclature
- 1% – https://en.m.wikipedia.org/wiki/Transmission_(mechanics)
- 1% – https://diabetestalk.net/ketosis/synthesis-of-ketones-from-carboxylic-acids
- <1% – https://www.encyclopedia.com/science-and-technology/chemistry/organic-chemistry/carbonyl-group
- <1% – https://www.britannica.com/science/aldehyde/Tautomerism
- <1% – https://www.britannica.com/science/aldehyde
- <1% – https://www.britannica.com/science/acetone
- <1% – https://www.brightstorm.com/science/chemistry/organic-chemistry/functional-groups/
- <1% – https://quizlet.com/99688524/chapter-1-organic-compounds-flash-cards/
- <1% – https://quizlet.com/130677478/ecology-chapter-2-flash-cards/
- <1% – https://celluloseether.com/
- <1% – https://byjus.com/chemistry/aldehydes-ketones/
- <1% – https://au.answers.yahoo.com/question/index?qid=20100904031714AAO5qQp
- <1% – https://answers.yahoo.com/question/index?qid=20070516092210AA8404T
- <1% – http://www.amrita.olabs.edu.in/?sub=73&brch=8&sim=141&cnt=1
- <1% – http://faculty.fiu.edu/~wnuk/CHM2210%20Fall%202008/SolomonsChapter%2012.pdf
- <1% – http://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/qual/9back6.htm
