Interesting Science Videos
Aldoses Definition
An aldose is a monosaccharide consisting of a carbon backbone and a carbonyl group at carbon-1, resulting in an aldehyde group.
- The general formula of aldoses is the same as most carbohydrates, Cn(H2O)n. The carbon atoms in the carbon backbone are each bonded to a hydroxyl group.
- All aldoses exhibit stereoisomerism as they have an asymmetrical carbon center. These compounds can exist in either L-form or D-form depending on the chirality of the asymmetric carbon.
- Aldoses with alcohol groups on the right are termed D-aldoses, whereas the aldoses with alcohol on the left are termed L-aldoses.
- Aldoses are polyhydroxy aldehydes that can also exist in a cyclic ring structure called hemiacetals. The cyclic structure can be seen in carbohydrates with more than 4 carbon atoms.
- Aldoses are often referred to by names that indicate their stereoisomerism, as many biological systems can only use a particular enantiomer of a carbohydrate.
- Aldoses can also tautomerize into ketoses through the dynamic process with an enol intermediate formation. The tautomerization is reversible, and the aldo-form is usually more stable than the enol-form.
Ketoses Definition
A ketose is a monosaccharide consisting of a carbon backbone and a carbonyl group within the backbone.
- The general formula for ketoses is the RCOR’ where the R is an alkyl group which can be the same or different from the other R’.
- All monosaccharide ketoses are reducing sugars as they can be tautomerized into aldehyde, which then undergoes oxidation. However, ketoses that are bound to glycosides are nonreducing sugars.
- The carbonyl group in ketoses is not present at the end of the chain, which results in a hemiketal cyclic ring structure in contrast to the hemiacetal ring structure in the aldehyde.
- The carbon atoms in ketoses are asymmetrical, resulting in different forms of sugars due to the chirality of the asymmetrical carbon. The L- and D- forms of ketoses can be defined by the position of the hydroxyl group on the carbon backbone.
- Ketoses can be differentiated from aldoses by Seliwanoff’s test. The test is based on the dehydration reaction, which is faster in ketoses, resulting in the faster test result.
7 Key Differences (Aldoses vs Ketoses)
| Characteristics | Aldoses | Ketoses |
| Definition | An aldose is a monosaccharide consisting of a carbon backbone and a carbonyl group at carbon-1, resulting in an aldehyde group. | A ketose is a monosaccharide consisting of a carbon backbone and a carbonyl group within the backbone. |
| Functional group | Aldoses have aldehyde as the functional group. | Ketoses have ketone as the functional group. |
| Also called | Aldoses are also called polyhydroxy aldehydes. | Ketones are also called polyhydroxy ketones. |
| Seliwanoff’s Test | Aldoses react slowly to Seliwanoff’s reagent and produce a light pink color. | Ketoses react with Seliwanoff’s reagent quickly and produce a deep cherry-red color. |
| Tautomerization | Aldoses can tautomerize into ketoses via enol intermediate formation. | Ketose can only tautomerize into aldoses if the carbonyl group is present at the end of the hydrocarbon chain. |
| Cyclic structure | Aldoses can exist in a cyclic form called hemiacetals. | The cyclic ketoses are termed hemiketals. |
| Examples | Glucose, ribose, arabinose, mannose are some of the examples of aldoses. | Fructose, ribulose, dihydroxyacetone are some of the examples of ketoses. |
Examples of Aldoses
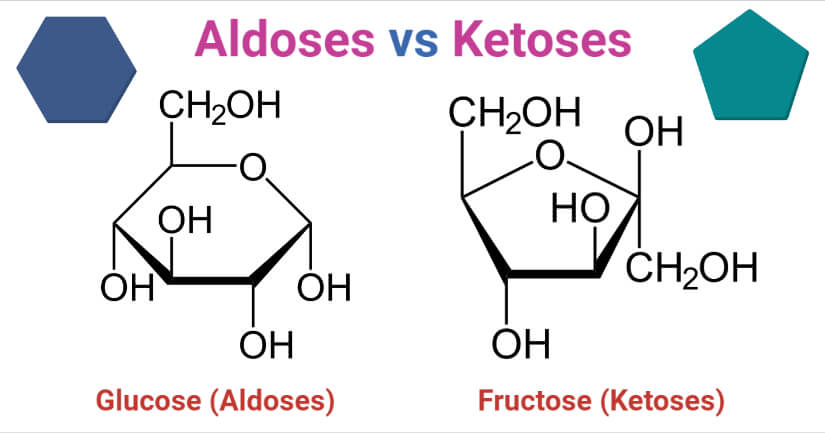
Glucose
- Glucose is an aldose monosaccharide sugar with the molecular formula C6H12O6, primarily produced by plants and algae by photosynthesis.
- Glucose is the most important source of energy in many organisms as it can be utilized by a large variety of organisms.
- It can also be stored in the form of polymer in plants as starch and animals as glycogen. The polymers are then broken down into glucose units during metabolism.
- The naturally occurring glucose exists in the D-glucose form, whereas the L-glucose can be produced synthetically for specific purposes.
- The D-glucose is more important than L-glucose as the biological systems have mechanisms to utilize D-glucose.
- Glucose is an aldohexose as it has six carbon atoms and an aldehyde group. The glucose molecule can exist either in an open-chain (acyclic) or ring (cyclic) form.
Examples of Ketoses
Fructose
- Fructose is a simple hexose sugar commonly found in plants and is one of the three dietary monosaccharides along with glucose and galactose.
- Fructose has a ketone functional group and the ring structure of fructose forms at the 2nd carbon position. The ring structure of fructose is a 5-carbon ring with an intramolecular hemiacetal structure.
- It is the most water-soluble of all the sugars that, together with glucose, forms a disaccharide structure like sucrose.
- Fructose derived from plant sources like sugar cane, maize, and beets is used to form high-fructose corn syrup with glucose as monosaccharides.
- The hemiketal structure of fructose is stabilized by the internal hydrogen-bonding resulting in the crystalline form. The crystalline form is called D-fructopyranose.
References and Sources
- 2% – https://www.difference.wiki/aldose-vs-ketose/
- 2% – https://en.wikipedia.org/wiki/Polyhydroxyaldehyde
- 1% – https://www.answers.com/Q/Most_soluble_sugar
- 1% – https://quizlet.com/49073732/carbon-chemistry-flash-cards/
- 1% – https://en.wikipedia.org/wiki/Ketose
- 1% – https://en.m.wikipedia.org/wiki/Ketone
- 1% – https://en.m.wikipedia.org/wiki/Aldose
- 1% – https://diabetestalk.net/blood-sugar/how-many-chiral-centers-are-there-in-the-open-chain-form-of-glucose-in-the-cyclic-form
- 1% – https://diabetestalk.net/blood-sugar/glucose-structure
- 1% – https://byjus.com/jee/fructose-structure/
- 1% – https://byjus.com/chemistry/structure-of-glucose-and-fructose/
- <1% – https://www.sciencedirect.com/topics/chemistry/glyceraldehyde
- <1% – https://www.ansaroo.com/question/why-is-d-glucose-more-common-than-l-glucose
- <1% – https://en.wikipedia.org/wiki/Energy
- <1% – https://en.m.wikipedia.org/wiki/Ketose
- <1% – https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Carbohydrates/Ketoses
- <1% – https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)/14%3A_Organic_Compounds_of_Oxygen/14.09%3A_Aldehydes_and_Ketones-_Structure_and_Names
