UV Spectroscopy- Definition, Principle, Parts, Uses
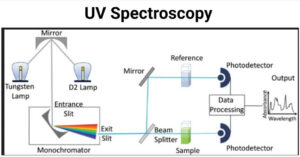
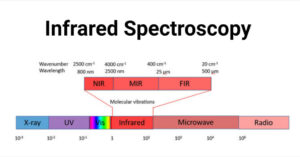
UV spectroscopy involves the promotion of electrons (n, σ, π) from the ground state to a higher energy state. In U.V. spectroscopy, the excitation of the electron occurs in the range of 200-800 nm. Principle of UV Spectroscopy When the … Read more