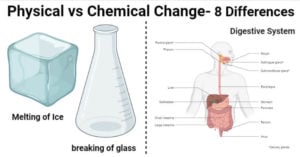
Physical vs Chemical Change- Definition, 8 Major Differences, Examples
Physical Change Definition A physical change is a process that changes the physical form of a substance but not its chemical composition. During a physical change, the molecules of a substance are rearranged, but the chemical composition of the substance … Read more