Interesting Science Videos
What is Cysteine?
Cysteine Definition
Cysteine is a non-essential crystalline sulfur-containing amino acid and is abbreviated as Cys. Cystine is one of the main five amino acids (arginine, cysteine, glutamine, tyrosine, and histidine) that are needed for human growth and for survival.
It is the main protein of skin, nails, and hair and is found in beta-keratin. It can be synthesized in the body from another amino acid methionine and serine. Cysteine has the main component for antioxidant properties in the form of glutathione.
Cysteine was discovered in 1810 by Wollaston isolated from animal horns. Until 1899, it is not considered as protein. Later, it was recognized as the important component required for the human body. It is the first amino acid to be discovered.
Structure of Cysteine
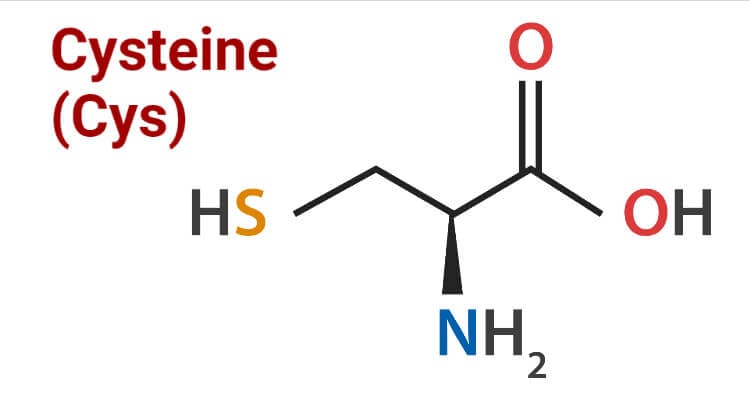
Cysteine contains sulfur in its structure as a thiol group. The high reactive capacity of the amino acid is due to the presence of the thiol group in the side-chain which also plays a vital role in operating biological functions in human beings. Thiol groups can undergo reduction reactions, therefore, cysteine can also undergo redox reactions. Oxidation of cysteine can produce a disulfide bond with another thiol. A disulfide bond or disulfide bridge is a single covalent bond derived from the coupling of thiol groups. The overall connectivity is C-S-S-C. Cysteine is also found in the body as cystine, which is made up of two cysteine molecules joined together. In proteins and peptides, two sulfur atoms are bonded together to make cysteine. Bonded sulfur atoms form the disulfide bridge that is the important factor in the function of skeletal and connective tissue. The thiol group of cysteine is a nucleophile.
Sources of Cysteine
It can be found in different foods including broccoli, egg yolks, garlic, oats, onions, poultry, yeasts, and wheat. Animal proteins contain a higher level of cysteine than plant proteins.
Physical Properties of Cysteine
- Solid dry powder
- Crystalline
- Molecular weight: 121.16
- Sulferous aroma
- Hygroscopic
- Oxidizes and decomposes slowly. It decomposes at 260 ºC.
- Glucogenic
- Water solubility: 28g/100 mL at 25 ºC.
- Heavy atom count: 7
- Isotope atom count: 0
- Topological polar surface area: 64.4 Angston
- Formal charge: 0
- Complexity: 75.3
- Covalently Bonded Count: 1
- Hydrogen Bond Donor Count: 3
- Defined Atom Stereocenter count: 1
- Undefined Atom Stereocenter count: 0
Chemical properties of Cysteine
- Molecular formula: C3H7NO2S
- LogP: -2.49
- pK1: 1.71, pK2: 8.33, pK3: 10.78
- Freely soluble in water, acetic acid, and ammonia.
- More stable in acid solutions as compared to slightly alkaline or neutral solutions.
- In neutral or slightly alkaline solutions, it is oxidized by air to cystine.
- Insoluble in ether, acetone, benzene, carbon tetrachloride.
- Melting point: 240 ºC.
- Specific optical rotation:+9.7º in 1M HCl, +13º in glacial acetic acid, +6.5º in 5N HCl
- Exists as a zwitterion.
- When heated, it emits toxic fumes of sulphur oxides or nitrogen oxides to decomposition
Biosynthesis of Cysteine
Oxidation of the thiol group present in cysteine residues forms disulfide bonds of proteins. L-serine and L-Methionine are the two main important components that are needed for cysteine biosynthesis. Methionine is the main component that synthesizes cysteine being converted to S-adenosyl methionine (SAM) which is then converted to homocysteine and then to cysteine. Moreover, cysteine sulfur is derived from methionine whereas carbon and nitrogen of cysteine are derived from L-serine.
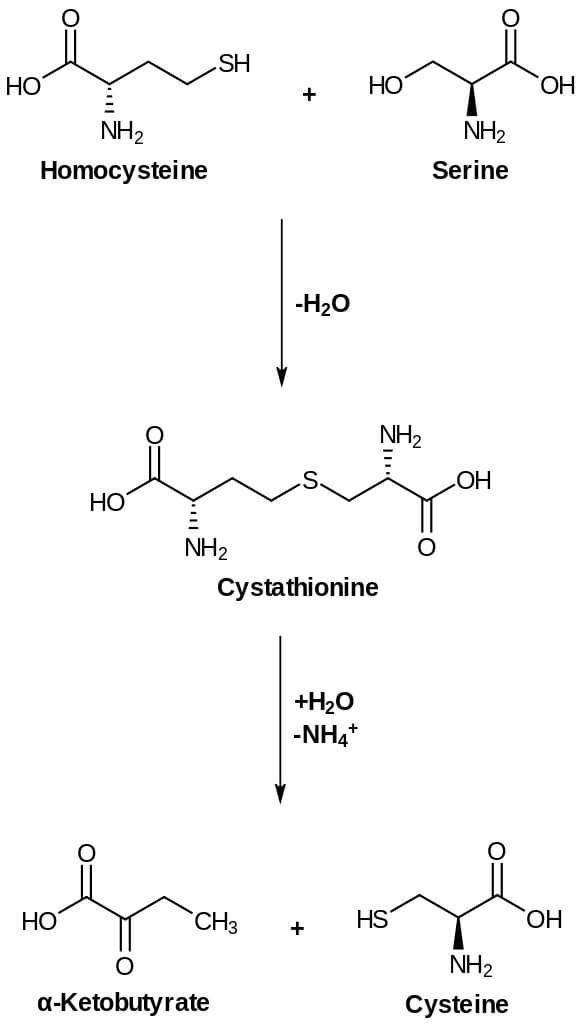
- Homocysteine formed from methionine is the main precursor for the synthesis.
- Cystathionine is formed when homocysteine condenses with serine. This reaction is catalyzed by PLP-dependent cystathionine synthase.
- The enzyme cystathioninase that is PLP dependent cleaves and deamination occurs. Deaminations result in cysteine and α-ketobutyrate from cystathionine.
Functions and Uses of Cysteine
- Cysteine plays important role in the metabolism of essential biochemical components like heparin, biotin, and coenzyme A.
- It helps in the detoxification of chemicals and heavy metals.
- It also protects cells from free radical damage.
- It also helps in the breakdown of extra mucus in the lungs. It is a useful therapy used to treat bronchitis by eliminating the extra mucus from the lungs.
- It is responsible for skin elasticity and texture.
- It is used as an antidote for acetaminophen toxicity.
- It is also used as a substitute for thioglycolic acid in permanent hair wave preparation.
- Cysteine can also be used to treat an oral infection like gingivitis, COPD, and metabolic and genetic defects.
Safety and Toxicity of Cysteine
- As cysteine is a brain excitotoxin, some people may have a loss of neural functions and cell death.
- A high dose of cysteine causes vomiting, diarrhea, nausea, and a drop in blood pressure.
References
- Eagle H, Piez AK and Oyama IV (1961).The biosynthesis of cysteine in human cell cultures. The Journal of Biological Chemistry. 236:1425-1428.
- Ismail IN, Hashim ZY, Jamal P, Othman R, and Salleh MH (2014). Production of cysteine: Approaches, challenges, and potential solution. International Journal of Biotechnology for Wellness Industries. 3: 95-101.
- Noelia Clemente Plaza, Manuel Reig García-Galbis, Rosa Maria Martinez-Espinosa. Molecules. 2018 Effects of the Usage of L-Cysteine (l-Cys) on Human Health (2018). 23: 575.
- From, www.restorativemedicine.org/library/monographs/cysteine.
- From, https://pubchem.ncbi.nlm.nih.gov/compound/Cysteine.
- Preston WG (2017). Cysteine: an essential inessential amino acid. 1-2.
