Interesting Science Videos
Ionic Bond Definition
The ionic bond is a type of chemical interaction or linkage as a result of electrostatic attraction between oppositely charged ions or atoms having different electronegativities.
- The ionic bond is one of the three major types of chemical bonds occurring between chemical units in order to reach a stable state.
- During the formation of an ionic bond, the transfer of electrons occurs from one chemical unit to another. As a result, charged ions are formed.
- The atoms that gain electrons during the formation of ionic bonds become negatively charged and are called anions. The atoms that lose electrons during the formation of ionic bonds become positively charged and are called cations.
Read Also: Cation vs Anion- Definition, 10 Major Differences, Examples
- Most commonly, metal ions act as cations, whereas nonmetal ions act as anions as a result of their electronic configuration.
- Ionic bonds are formed as the result of electrostatic forces of attraction and repulsion between opposite charges and similar charges respectively. The resulting product formed as a result of ionic bonding is called an ionic compound.
- Ionic bonds are also observed in neutralization reactions of acid and base, and thus, these compounds are mostly termed salts.
- An ideal ionic bonding with the complete transfer of electrons from one chemical moiety to another doesn’t exist because all ionic compounds tend to have some degree of covalent bonding as a result of electron sharing.
- The term ionic bond is thus used when the ionic character of the bond is greater than the covalent character.
- The strength of the ionic bond depends on the electrovalency of the atoms, which further depends on the electronic configuration of the atoms.
What are electrovalent bonds?
- Electrovalent bonds are chemical bonds formed due to the transfer of electrons from the electron-rich chemical unit to the electron-deficient chemical unit, resulting in charged ions. Electrovalent bonds are also called ionic bonds.
Condition for Ionic Bonding
The following are the conditions for the formation of ionic bonding between chemical substances;
- The two elements or ions involved in the formation of the ionic bond should be of opposite charges. The most common types of elements that form ionic bonds are metals and nonmetals.
- One of the atoms (metal) involved in the bond formation must have a low value of ionization or low electron affinity so that it can lose electrons to form positively charged ions (cations) easily.
- The next atom should have high ionization energy or high electron affinity so that it can gain electrons easily and form negatively charged ions (anions).
- The two atoms involved in ionic bond formation should have a difference of electronegativity greater than 1.7.
- The ionic compound formed as a result of the bonding should have high lattice energy in order to be stable.
- The ionic property of the bond should be higher than the covalent property.
Ionic bond properties
Ionic bonds and ionic compounds have the following properties;
1. State
- Ionic compounds exist in solid-state at room temperature as the bond holding the atoms is the strong electrostatic force of attraction.
- The ionic compounds have high lattice energy which makes them very stable in the solid-state.
2. Solubility
- Most of the ionic compounds are soluble in polar solvents like water, but they remain insoluble in non-polar solvents like benzene.
- The solubility is due to the breakage of ionic bonds which can only be brought by polar solvents like water. The ionic dipole interaction results in the hydration of ions, resulting in solubility.
3. Boiling and melting point
- As the force of attraction in the ionic bond is strong, ionic compounds have high boiling and melting point as they require a large amount of heat energy to break those bonds.
4. Density
- The strong force of attraction between the chemical units involved in ionic bonds enables the ions to be arranged orderly in a close-packed crystal form.
- The crystal form of the compounds increases the density of compounds.
5. Brittleness
- The arrangement of the ions in the form of crystal lattice also increases their brittleness.
- The hammering of these compounds brings the ions closer together in the lattice resulting in the regeneration of repulsive forces. The repulsive forces, thus, increase the brittleness of the compound.
6. Non-directional
- Ionic bonds are non-directional in nature as the force of attraction between the oppositely charged ions acts equally in all directions.
- The ionic compounds are also non-directional in nature and do not possess isomerism.
7. Electrical conductivity
- Ionic compounds in the aqueous and molten state dissociated into charged ions. The charged ions can carry electrical charges which increase electrical conductivity.
- These compounds cannot, however, conduct electricity in the solid-state as the ions are not free in the solid-state.
How are ionic bonds formed?
- Ionic bonds are formed between atoms that have an electronegativity difference of 1.7 or higher.
- When such atoms come closer, the difference in electronegativity causes an unequal sharing of electrons so that one atom completely loses an electron while the other accepts the electron.
- Ionic bond formation occurs through redox reaction when the atom with low ionization energy gives one or more electrons to reach a stable electron configuration. The resulting chemical units are termed cations.
- The atom of another element with high electron affinity accepts the electron from the other atom to receive a stable electron configuration. The resulting chemical species are termed anions.
- The strength of ionic bonds depends on the electrovalency of the atoms as the bonds are formed in order to achieve the electronic configuration of noble gases in different blocks.
- During the formation of ionic bonds, strict ratios are observed between anions and cations as the ionic compounds follow the rules of stoichiometry.
- The formation of ionic bonds occurs only if the overall change in the energy of the system is favourable.
- The formation of cations or the removal of electrons is an endothermic process which raises the energy of the system. The energy, however, is lowered during the subsequent attraction between the ions and the acceptance of electrons by the anions.
Examples of Ionic Bonds
1. Ionic bonding in NaCl
- In NaCl, the ionic bond is formed between the metal ion, Na+, and the non-metal ion, Cl–.
- The sodium atom has the configuration of 1s2 2s2 2p6 3s1, indicating that it has a single valence electron in the outermost shell.
- The atom tends to lose one electron in order to achieve a stable electronic configuration.
- The chlorine atoms being a non-metal has the configuration of 1s1 2s2 2p6, and it tends to accept one electron in order to achieve a stable electronic configuration.
- During the formation of the bond, the sodium atom loses one electron, resulting in the formation of sodium cation whereas the chlorine atom accepts one electron to form a chlorine anion.
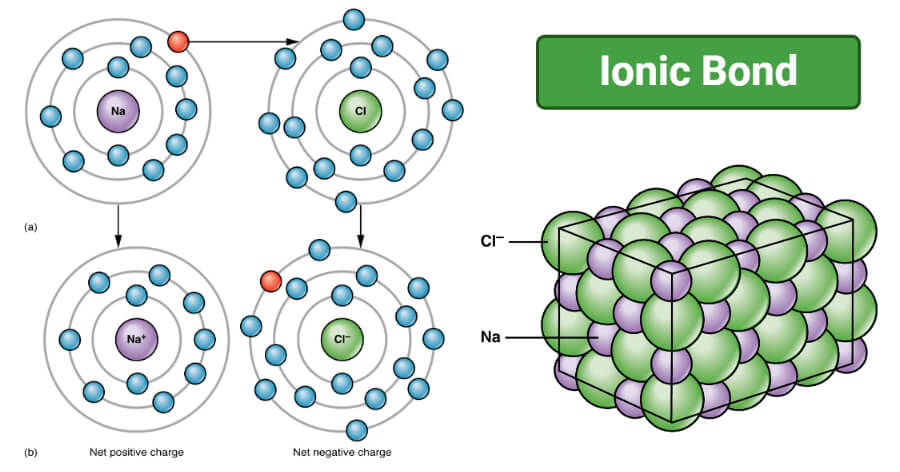
Figure: Ionic Bonding (a) Sodium readily donates the solitary electron in its valence shell to chlorine, which needs only one electron to have a full valence shell. (b) The opposite electrical charges of the resulting sodium cation and chloride anion result in the formation of a bond of attraction called an ionic bond. Image Credit: Openstax.
2. Ionic bonding in MgO
- The bond between the magnesium atom and the oxygen atom in the compound MgO is also an ionic bond as it involves the transfer of electrons from magnesium to oxygen.
- The magnesium atom has two electrons in its outermost orbit, which results in low ionization energy. In turn, the oxygen atom requires two electrons to attain a stable electronic configuration.
- The transfer of electrons between the two atoms is possible and results in the formation of magnesium cation and oxygen anion.
Applications of Ionic Bonds
- There are different applications of ionic compounds like NaCl and CaCO3 in different areas. The ionic bonds enable the compounds to exist in the solid-state increasing their solubility and conductivity.
- The tertiary and quaternary structures of proteins are stabilized by ionic, which helps maintain the shape of the protein.
- Ionic bonds are also involved in the formation of the shape of chromosomes that holds the atoms of different organic compounds together.
- Ions in cells help maintain the cell potential and other important functions like cell signaling and muscle contraction.
- Ionic bonds are present between organic compounds that help maintain the shape of the cell and perform various catalytic reactions and neuron functions.
References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 2.2, Noncovalent Bonds. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21726/
- 1% – https://www.meritnation.com/ask-answer/question/give-reasons-1-in-the-formation-of-mgo-the-magnesium-atom-a/chemical-bonding/5454789
- 1% – https://www.icserankers.com/2020/11/icse-solutions-for-chapter2-chemical-bonding-class10-chemistry.html
- 1% – https://www.cliffsnotes.com/study-guides/chemistry/chemistry/chemical-bonding/ionic-bonds
- 1% – https://www.britannica.com/science/ionic-bond
- 1% – https://www.bbc.co.uk/bitesize/guides/ztc6w6f/revision/4
- 1% – https://socratic.org/questions/why-don-t-ionic-compounds-have-electrical-conductivity-as-a-solid-but-they-do-as
- 1% – https://en.wikipedia.org/wiki/Ionic_interaction
- 1% – https://en.wikipedia.org/wiki/Electrovalency
- 1% – https://answers.yahoo.com/question/index?qid=20110211132151AAajTjs
- 1% – https://answers.yahoo.com/question/index?qid=20090823012150AAqIgN6
- <1% – https://www.zmescience.com/other/feature-post/difference-ionic-covalent-bonds-0423/
- <1% – https://www.wisegeek.com/what-is-the-electron-affinity.htm
- <1% – https://www.weknowtheanswer.com/q/the-process-of-ionisation-is-endothermic-or-exothermic
- <1% – https://www.topperlearning.com/answer/why-ionic-compounds-are-soluble-in-polar-solvents-and-insoluble-in-non-polar-solvents/rv7q9l0cc
- <1% – https://www.thoughtco.com/cytoskeleton-anatomy-373358
- <1% – https://www.quia.com/jg/87062list.html
- <1% – https://www.chemguide.co.uk/atoms/structures/ionicstruct.html
- <1% – https://www.bbc.co.uk/bitesize/guides/ztc6w6f/revision/3
- <1% – https://www.adichemistry.com/general/chemicalbond/ionicbond/ionic-bond.html
- <1% – https://saylordotorg.github.io/text_introductory-chemistry/s13-02-electron-transfer-ionic-bonds.html
- <1% – https://quizlet.com/49046914/biology-101-chapters-1-5-8-flash-cards/
- <1% – https://pediaa.com/difference-between-electrovalent-and-covalent-bond/
- <1% – https://igcseandialchemistry.com/ionic-bond-formation/
- <1% – https://en.wikipedia.org/wiki/Ionic_bonding
- <1% – https://chemistry.stackexchange.com/questions/9222/why-are-bonds-ionic-when-the-electronegativity-difference-between-bonded-atoms-i
- <1% – https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Beginning_Chemistry_(Ball)/09%3A_Chemical_Bonds/9.3%3A_Electron_Transfer_-_Ionic_Bonds
- <1% – https://biodifferences.com/difference-between-covalent-metallic-and-ionic-bonds.html
- <1% – http://pressbooks-dev.oer.hawaii.edu/chemistry/chapter/ionic-bonding/
- <1% – http://chemistry.elmhurst.edu/vchembook/143Amgoxide.html
