Interesting Science Videos
Who is Mikhail Vasilyevich Lomonosov?
- Mikhail Vasilyevich Lomonosov (November 10, 1711- April 15, 1765) is a Russian scientist, polymath, and writer who is widely known for his contributions in chemistry by the discovery of the law of conservation of mass.
- Besides, he is also known for his contributions to astronomy as he was associated with the discovery of the atmosphere of Venus.
- Even within chemistry, Lomonosov discovered the freezing point of mercury and carried out various experiments with it.
Law of conservation of mass definition
The law of conservation of mass states that in a chemical reaction, the total mass of reactants involved is always equal to the total mass of products produced so that the overall mass of the system remains constant.
- In other words, the mass within a closed system remains the same as there is no increase or decrease in the quantity of matter.
- The law is equivalent to the law of conservation of energy as energy and mass are convertible quantities.
- The law of conservation of mass determines the mathematical relationship between the mass of the reactants and the mass of the products.
- The first understanding of the conservation of mass originated with the discovery by the French chemist Lavoisier in 1774 that mass can neither be created nor be destroyed.
- The law was later put forward by the Russian scientist Mikhail Vasilyevich Lomonosov. It is thus also known as the Lomonosov-Lavoisier Law.
- The law is in accordance with Dalton’s atomic theory as he stated that the total number of atoms of chemical species involved in a chemical reaction remains conserved.
Read Also: Dalton’s Atomic Theory- Postulates, Merits, Limitations
Law of conservation of mass formula
The law of conservation of mass can be expressed in a mathematical formula by using the continuity equation. The formula is in the differential form and is often used in fluid mechanics and continuum mechanics.
The formula for the law of conservation of mass can be written as:
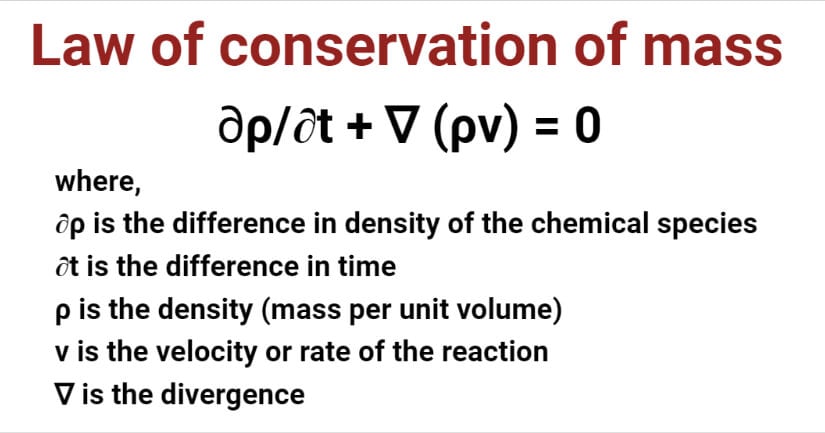
∂ρ/∂t + ∇ (ρv) = 0
where,
∂ρ is the difference in density of the chemical species
∂t is the difference in time
ρ is the density (mass per unit volume)
v is the velocity or rate of the reaction
∇ is the divergence
Law of conservation of mass equation
The law of conservation of mass can be expressed in terms of the equation if we consider a chemical reaction:
A + B → C + D
If X grams of A combines with Y grams of B in a chemical reaction to produce P grams of C and Q grams of D, the law of conservation of mass can be expressed as;
X + Y = P + Q
Thus, the total mass of the reactants = total mass of the products.
Law of conservation of mass examples
The law of conservation of mass can be observed in various closed systems, both chemical as well as natural. Some of the examples of the law are:
Combustion
- One of the most easily observed examples of the law of conservation of mass is the process of combustion.
- The reactants involved in the combustion process are wood and oxygen, whereas the products are carbon dioxide, water vapor, and ashes.
- During this process, the mass of the reactants is equal to the mass of the products as it follows the law of conservation of mass.
The reaction between AgNO3 and NaCl
- The reaction between AgNO3 and NaCl is used as a means of experimental verification of the law of conservation of mass.
- During the process, AgNO3 and NaCl are taken two limbs of an H-shaped tube. The tube is weighted with the chemical species.
- The two solutions are then mixed by shaking the tube, and the reaction is allowed to happen.
- The product formed in the reaction is in the form of a curdy white precipitate of AgCl. The tube is then weighed again to determine the total mass of the products.
- During the process, no change in weight is observed after the chemical reaction.
AgNO3 + NaCl → AgCl + NaNO3
Exceptions to the Law of conservation of mass
- Even though many chemical reactions follow the law of conservation of mass, there are some exceptions to the law.
- The law has its drawbacks as in some cases, the mass of a system is not constant due to the conversion of mass into energy.
- According to Einstein’s mass-energy relation, he stated that mass and energy are interconvertible to one another and thus, in some chemical reactions, some mass might be converted into energy.
- One of the examples of such exceptions is a nuclear reaction. In a nuclear reaction, the mass of the reactants is not equal to the mass of the products as some mass is converted to energy.
- Thus, the law of conservation of mass has been redefined in modern chemistry as the total mass and energy during a chemical reaction always remains constant.
The Law of conservation of mass (Video By TED-Ed)

References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors, Pvt. Ltd.
- 8% – https://courses.lumenlearning.com/introchem/chapter/the-law-of-conservation-of-mass/
- 7% – https://study.com/academy/lesson/the-law-of-conservation-of-mass-definition-equation-examples.html
- 2% – https://en.wikipedia.org/wiki/Mass_conservation
- 2% – http://panchbhaya.weebly.com/uploads/1/3/7/0/13701351/conservation_of_mass_lab_exemplar.pdf
- 1% – https://www.youtube.com/watch?v=2S6e11NBwiw
- 1% – https://scienceforum.ru/2020/article/2018018706
- 1% – https://qsstudy.com/physics/mass-energy-relation
- 1% – https://madelineisn.weebly.com/conservation-of-mass-lab.html
- 1% – https://flexbooks.ck12.org/cbook/ck-12-middle-school-physical-science-flexbook-2.0/section/5.13/primary/lesson/reactants-and-products-ms-ps
- 1% – https://byjus.com/physics/law-of-conservation-of-mass/
- 1% – http://www.sfu.ca/~mbahrami/ENSC%20283/Solution%20manual/Chapter1_SM.pdf
- <1% – https://www.britannica.com/science/combustion
