The sulfur cycle is a biogeochemical process in which sulfur circulates around the Earth’s atmosphere, soil, water, and living organisms.
Sulfur is the fifth most prevalent element in the universe, the fifteenth the most abundant in the earth’s crust, and is required for life. Sulfur is necessary for biological processes and is found in some amino acids, such as cysteine, which is vital in protein structure. Sulfur is introduced to terrestrial (i.e., land) ecosystems by the precipitation of weak sulfuric acid, direct fallout from the atmosphere, weathering of sulfur-containing rocks, and geothermal vents.
What is Sulfur Cycle?
The sulfur cycle is a biogeochemical cycle made up of several processes that allow sulfur to move through different sources such as the atmosphere, biosphere, and lithosphere. The process begins with the weathering of rocks, which releases sulfur compounds into the soil. Microbial transformations, plant assimilation, breakdown, and air reactions complete the cycle. The sulfur cycle is essential for the formation of vital biomolecules including amino acids and vitamins, as well as the regulation of atmospheric and aquatic chemistry.
The cycle includes both biotic (living creatures) and abiotic (non-living) components. The cycle begins with rocks eroding and releasing sulfur into the atmosphere. Sulfur then combines with oxygen in the air, forming sulfate, which plants and microbes absorb. They convert it into organic forms and distribute it throughout the food chain. The cycle is restarted when the animals break down and release the sulfur back into the atmosphere.
[Image source: https://www.britannica.com/science/sulfur-cycle#/media/1/572740/111671]
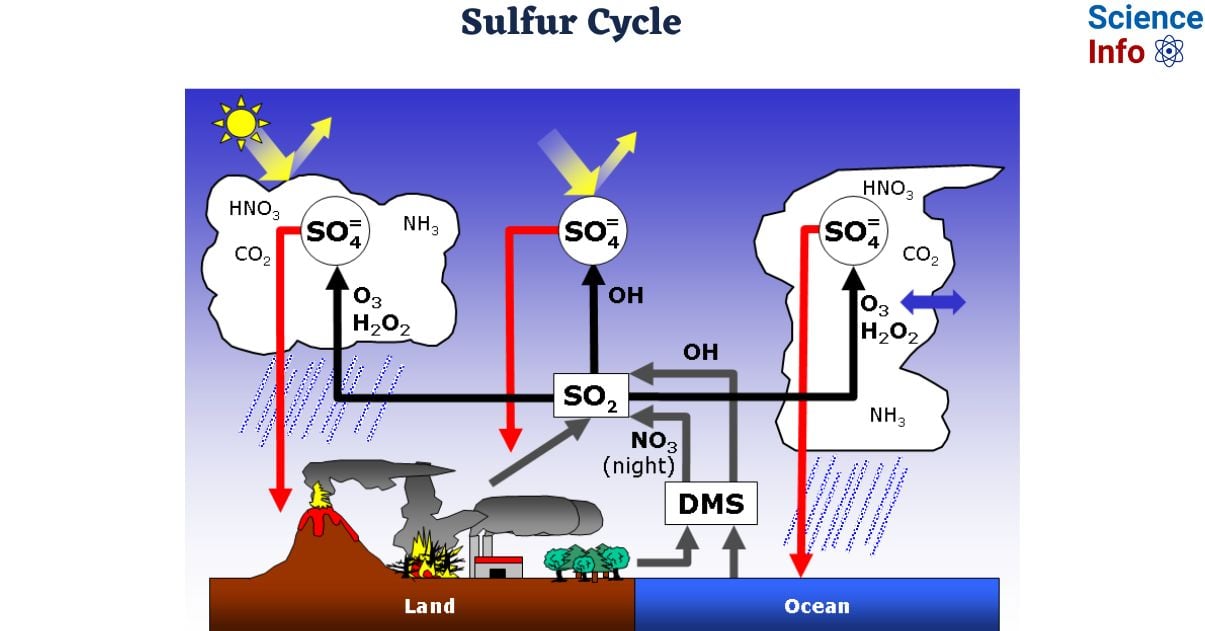
- On land, sulfur is deposited in four principal ways: precipitation, direct atmospheric fallout, rock weathering, and geothermal vents
- Sulfur dioxide (SO2) is prevalent in the atmosphere, and when rain falls, sulfur is dissolved as weak sulfuric acid (H2SO4). In addition, sulfur can fall straight from the atmosphere through a process known as fallout.
- he weathering of sulfur-containing rocks releases sulfur into the soil. These rocks are formed from ocean sediments that have been transferred on land as a result of geologic rising.
- Terrestrial ecosystems use soil sulfates (SO4−) and release hydrogen sulfide (H2S) gas into the atmosphere when the organisms die and decompose.
- Sulfur enters the ocean through runoff from the land, air fallout, and underwater geothermal vents. Some ecosystems rely on chemoautotrophs, which use sulfur as a biological energy source. This sulfur then feeds marine ecosystems in the form of sulfate.
- Human actions have significantly altered the balance of the global sulfur cycle. The combustion of huge amounts of fossil fuels, particularly coal, emits higher levels of hydrogen sulfide gas into the atmosphere.
Steps of Sulfur Cycle
The sulfur cycle consists of a series of aerobic and anaerobic reactions of sulfur-containing compounds. It has a critical role in cellular and ecosystem processes that regulate biological carbon transfers and other biogeochemical cycles. The different stages of the sulfur cycle include:
Decomposition of Organic Compound
Dead plants and animals contain sulfur in the form of organic compounds, which are broken down by decomposer microbes during the process of decomposition. This breakdown liberates sulfur-containing molecules, including amino acids from proteins. Desulfotomaculum bacteria reduces sulfates to hydrogen sulfide.
SO42- + Organic matter → H2S + CO2 + H2O
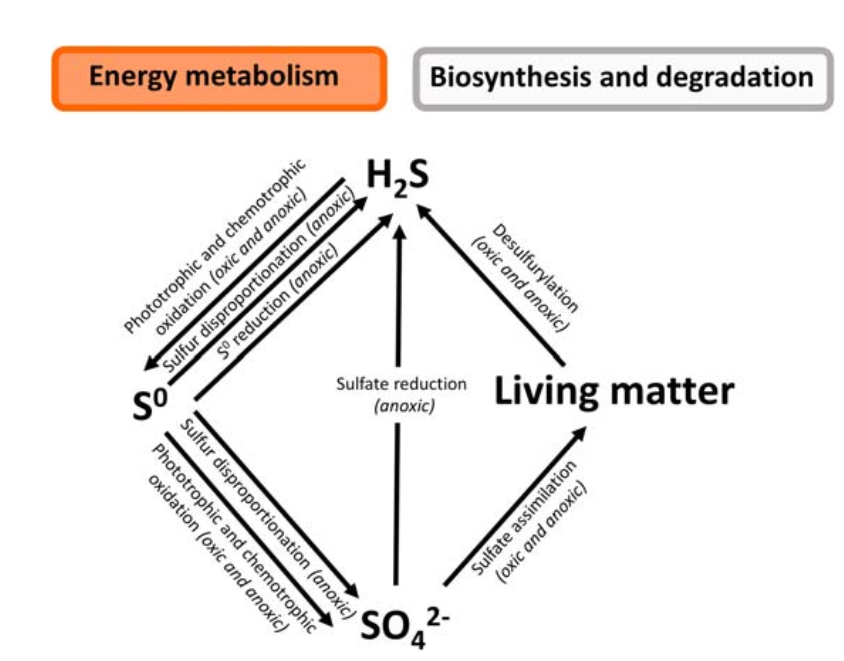
Oxidation of hydrogen sulfide to elemental sulfur
Microbial and chemical mechanisms reduce hydrogen sulfide (H₂S) to elemental sulfur (S⁰). This oxidation is carried out by photosynthetic bacteria, including those from the Chlorobiaceae and Chromatiaceae families. There are two types of lithoautotrophic sulfur-oxidizing bacteria: phototrophic prokaryotes, which employ sulfur compounds as electron donors to fix CO2 in light. Chemotrophic sulfur-oxidizing prokaryotes use energy from oxidizing sulfur compounds with oxygen, nitrate, or Mn(IV) oxide to fix CO2. The elemental sulfur often stored in polysulfides.
H2S + Oxygen → S0 + H2O
Elemental sulfur is found in several environments, including volcanoes, sulfidic springs, deep-sea hydrothermal vents, hydrocarbon seeps, salt marshes, and marine sediments. Elemental sulfur can be generated through abiogenic processes when sulfide is oxidized by molecular oxygen, perhaps accelerated by oxidized metals.
Oxidation of Elemental Sulfur to Sulfates
Sulphur exists in the soil in its elemental form, which plants cannot directly use. Chemolithotrophic bacteria, such as Thiobacillus, convert elemental sulfur into sulfates (SO₄²⁻). This reaction releases sulfur in a form that plants can absorb.
S0 + Oxygen → SO42-
Reduction of Sulfates
The cation of certain bacteria, such as Desulfovibrio desulfuricans, converts sulfates in the soil back to hydrogen sulfide. The reduction process has two steps: first, sulfates are transformed to sulfites (SO⁻), followed by further reduction to hydrogen sulfide. Sulfate reduction produces hydrogen sulfide (H2S), which is then released into the atmosphere, completing the cycle.
SO42- + Organic matter → H2S + CO2 + H2O
Sulfate-reducing prokaryotes are commonly found in anoxic environments and use sulfate as a terminal electron acceptor for organic compound breakdown. Deltaproteobacteria, including Desulfovibrio, Desulfobulbus, and Desulfonema, are a class of important sulfate reducers. The Desulfobacteraceae family is particularly active and numerous. Sulfate reducers are present in Gram-positive bacteria, Thermodesulfobacteria, Nitrospira, and Archaeoglobus archaea.
[Image source: http://dx.doi.org/10.2166/9781789060966_0055]
Dissimilative Sulfate Reduction
Dissimilative sulfate reduction is a metabolic process in which bacteria employ sulfate (SO₄²⁻) as a terminal electron acceptor to create energy by oxidizing diverse substrates. This process is essential in the sulfur cycle, especially in anaerobic settings.
Step 1: Sulfate is quite stable and reacts with ATP to create adenosinephosphosulfate (APS).
SO4 2- + ATP → APS + PPi
Step 2:The hydrogenase enzyme splits molecular hydrogen (H₂) and uses the electrons to reduce the sulfur atom in APS, resulting in sulfite (SO₃²⁻). This phase involves an intermediary electron carrier, cytochrome c3, which is found in dissimilative sulfate reducers.
APS + H2 → SO32- + AMP + H2O
Step 3: Sulfite is converted to hydrogen sulfide (H₂S) by adding electrons from molecular hydrogen.
SO32− +6H+ + 6e− → H2S + H2O + 2OH−
Sulfur Cycle Processes in Atmosphere
Sulfur in the atmosphere is mostly found as sulfur dioxide (SO₂). Anthropogenic activities, primarily the combustion of fossil fuels, account for the majority of atmospheric SO₂ emissions. Volcanic eruptions play a key role in increasing atmospheric SO₂ levels.
Hydrogen sulfide (H₂S) is another important sulfur-containing gas in the environment. The primary source of atmospheric H₂S is microbial breakdown of organic materials from deceased and decaying organisms. These microbial processes take place in both terrestrial and aquatic settings under anaerobic circumstances.
Microbes release H₂S during anaerobic breakdown, which is then oxidized in the environment to produce SO₂. Microorganisms that produce H₂S by sulfate reduction are often anaerobic. They convert oxidized forms of sulfur to H₂S. Notable sulfate-reducing bacteria involved in this process include Desulfovibrio desulfuricans, Desulfovibrio vulgaris, Thermodesulfovibrio yellowstonii, Desulfotomaculum nigrificans, and Desulfobacula toluolica.
Sulfur Cycle Processes in Biosphere
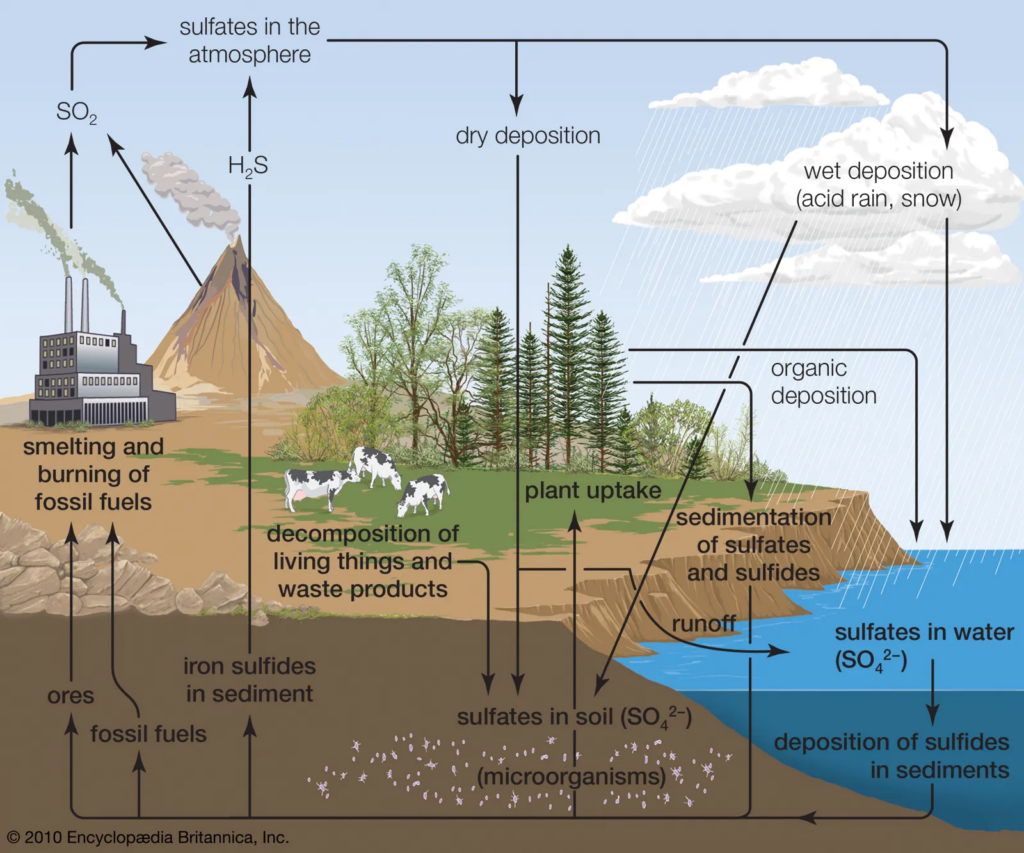
Sulfur predominantly enters the biosphere via two routes: atmospheric deposition and rock weathering. Sulfur eventually flows into the soil and then to the ocean via both routes.
Sulfur dioxide (SO₂) helps produce clouds by increasing the number of droplets and decreasing their size. Sulfur aerosols eventually descend from the atmosphere and settle into the biosphere.
SO₂ dissolves in rainwater, generating mild sulfuric acid that contributes sulfur to the soil during precipitation.
The chemical weathering process that occurs during soil formation (pedogenesis) transfers sulfur from rocks into the soil and water systems. Some sulfur released during weathering is transformed to sulfate and may be released back into the atmosphere.
Sulfur Cycle Processes due to Plant and Animal Uptake
When sulfur reaches the terrestrial and aquatic biospheres, it is absorbed by plants and microbes. Green sulfur bacteria are photoautotrophic bacteria that use sulfur for energy. Other bacteria in the soil help to make sulfur available to plants, allowing it to be absorbed with water from the soil. Sulfur is absorbed by living creatures and used to produce biomolecules such as proteins and nucleotides. In the ocean ecosystem, chemoautotrophic microbes use sulfur to generate organic chemicals such as sulfates.
Lithification & Release
The sulfur in the biosphere then circulates through the food chain as consumers feed on producers, eventually reaching the microbial chains. The sulfur that does not circulate falls into the depths of terrestrial and marine ecosystems, where it remains in the mixed form (FeS) in rocks. The sulfur in the food chain then decomposes, transforming sulfate into sulfides that can be recycled to the atmosphere. Sulfur-reducing bacteria convert organic forms of sulfur into inorganic forms such as hydrogen sulfide (H2S), which is then reduced to sulfur. The sulfur in the lithosphere is also released into the atmosphere as a result of volcanic activity.
Sulfur Cycle Reservoirs and Sources
- Atmospheric gas: Sulfur is found in the environment as gases like SO₂ and H₂S.
- Volcano Explosion: Released from volcanic activity sulfur is present in the atmosphere in the form of sulfur dioxide (SO₂) and hydrogen sulfide (H₂S).
- Water bodies: Sulfur can be found in various forms in aquatic settings such as seas, lakes, and rivers, known as the hydrosphere. In anaerobic conditions, hydrogen sulfide is present, whereas sulfate is found in aerobic water.
- Soil: Soil contains sulfur in the form of organic matter, sulfides, and sulfate, and microbiological activity in the soil contributes to sulfur transformations.
- Rocks and Minerals (Sedimentary Rocks): Sulfur is stored in various minerals, including pyrite, gypsum (CaSO₄·2H₂O), and anhydrite (CaSO₄). Sulfur is deposited in sediments as sulfide minerals and organic sulfur compounds.
- Fossil fuel combustion: Fossil fuels, such as coal and oil, can store sulfur. The combustion of fossil fuels releases sulfur dioxide (SO2) into the atmosphere.
- Living Organisms: Sulfur can be found in nucleic acid, amino acids, proteins, and other macromolecules.
- Soil Organic Matter: Sulfur is preserved in soil as organic matter, which is decomposable and recyclable.
- Deep Earth: Sulfur can be found in the Earth’s mantle and crust.
Importance of Sulfur Cycle
- Understanding the functioning of many biomolecules is essential for a thorough understanding of their activities, and this is why the sulfur cycle is so important.
- Sulfur is a key component of amino acids like cysteine and methionine. Sulfur forms key component of biomolecules like nucleic acids, and vitamins
- The sulphur cycle keeps sulphur concentrations in balance across the planet’s numerous reservoirs, helping to preserve the planet’s habitability and the ecological system that supports life.
- The availability of several other elements is also impacted by the sulfur cycle because sulfur is commonly found in nature in combination with other elements like iron, phosphorus, nitrogen, and so forth
- Numerous biological processes make up the terrestrial portion of the sulfur cycle, and each one is crucial in raising the quantity of sulfur that is available for microbial and plant life to use.
- The chemical energy is changed into a number of different forms by the chemoautotrophic sulfur bacteria that are a part of the food chain, which eventually increases the amount of biomass on the planet.
- The physiology of the different microorganisms engaged in the process of sulphur conversion can be understood through the perspective of the sulfur cycle.
- Researching the sulfur metabolism of pathogenic bacteria such as Mycobacterium TB is crucial for a variety of reasons related to medical problems being treated. One of the main sources of energy for these bacteria is sulfur.
- Since it is also in charge of the sulphur compounds’ regeneration, the sulphur cycle’s mineralization process is a naturally occurring way to get rid of waste.
- Sulfur-containing chemicals aid in the process of cellular detoxification, protecting organisms from oxidative stress and environmental pollutants.
- The sulfur cycle in the oceans is critical for marine ecosystems because it undergoes numerous transformations in seawater and changes the chemical composition of the ocean, which affects marine life.
Impact of Human on Sulfur Cycle
Human combustion activities account for around one-third of all sulfur compounds and 99% of the SO2 that reaches the troposphere. The combustion of coal and oil for electricity production, as well as the smelting of metal-bearing ores, have been major sources of SO2 emissions into the environment. Human actions have significantly altered the balance of the global sulfur cycle.
Without human intervention, sulphur would remain trapped in rocks for millions of years, only to be released after being uplifted by tectonic events and subjected to erosion and weathering processes. Instead, drilling, pumping, and burning are steadily increasing. The amount of sulfate settling in the most contaminated areas has increased thirtyfold.
- The combustion of vast quantities of fossil fuels, particularly coal, emits large amounts of hydrogen sulfide gas into the atmosphere, resulting in acid rain. Acid rain is corrosive rain that causes damage to aquatic ecosystems and the natural environment by decreasing the pH of lakes, killing many of the resident species; it also affects the man-made environment through the chemical corrosion of buildings.
- Artificial fertilizers can impact soil fertility, plant development, and microbial activity.
Petroleum refining and industrial operations also produce sulfuric acid and sulfur dioxide in the environment. - Industrial society, along with a fast-growing global population and increased mechanization of agriculture and forestry, transforms vast regions of previously undisturbed environment, altering the terrestrial biosphere.
Video on Sulfur Cycle
References
- https://microbenotes.com/sulfur-cycle/
- A biochemical view on the biological sulfur cycle October 2020 DOI: 10.2166/9781789060966_0055 In book: Environmental technologies to treat sulfur pollution: principles and engineering
- https://kegriver.com/soil-science-101-the-sulfur-cycle/
- Klotz MG, Bryant DA, Hanson TE. The microbial sulfur cycle. Front Microbiol. 2011;2:241. Published 2011 Dec 2. DOI:10.3389/fmicb.2011.00241
- https://courses.lumenlearning.com/wm-biology2/chapter/the-sulfur-cycle/
- https://www.chemistryworld.com/features/the-secrets-of-the-sulfur-cycle/4015331.article
- https://unacademy.com/content/upsc/study-material/general-awareness/sulphur-cycle-definition-steps-diagram-importance-and-facts/
- Muyzer, G., Stams, A. The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol6, 441–454 (2008). https://doi.org/10.1038/nrmicro1892
- https://www.britannica.com/science/sulfur-cycle
