Spectroscopic techniques use light to interact with matter, probing certain aspects of a sample to learn about its consistency or structure. Light is electromagnetic radiation, a phenomenon with varying energies that can be used to examine various molecular properties. The study of how electromagnetic radiation interacts with matter as a function of the radiation’s wavelength or frequency is known as spectroscopy.
The development of the most fundamental theories in physics, such as quantum mechanics, special and general theories of relativity, and quantum electrodynamics, has relied heavily on spectroscopic analysis. A spectroscopy experiment involves passing electromagnetic radiation of a specific wavelength range from a source through a sample containing substances of interest, resulting in absorption or emission.
Interesting Science Videos
Principle of Spectroscopic techniques
The term “spectroscopy” refers to a wide range of techniques that employ radiation to learn about the structure and properties of matter.
The underlying idea behind all spectroscopic techniques is to radiate a beam of electromagnetic radiation onto a sample and see how it reacts to it.
Spectroscopy is most commonly used to identify and explain the elements and compounds of atoms and molecules. They are determined by evaluating the radiant energy absorbed or emitted by the sample or object. In this case, a beam of electromagnetic radiation such as infrared rays, UV rays, and so on is passed on the sample, and the response of the sample is measured using the wavelength of the electromagnetic spectrum applied from an external energy source.
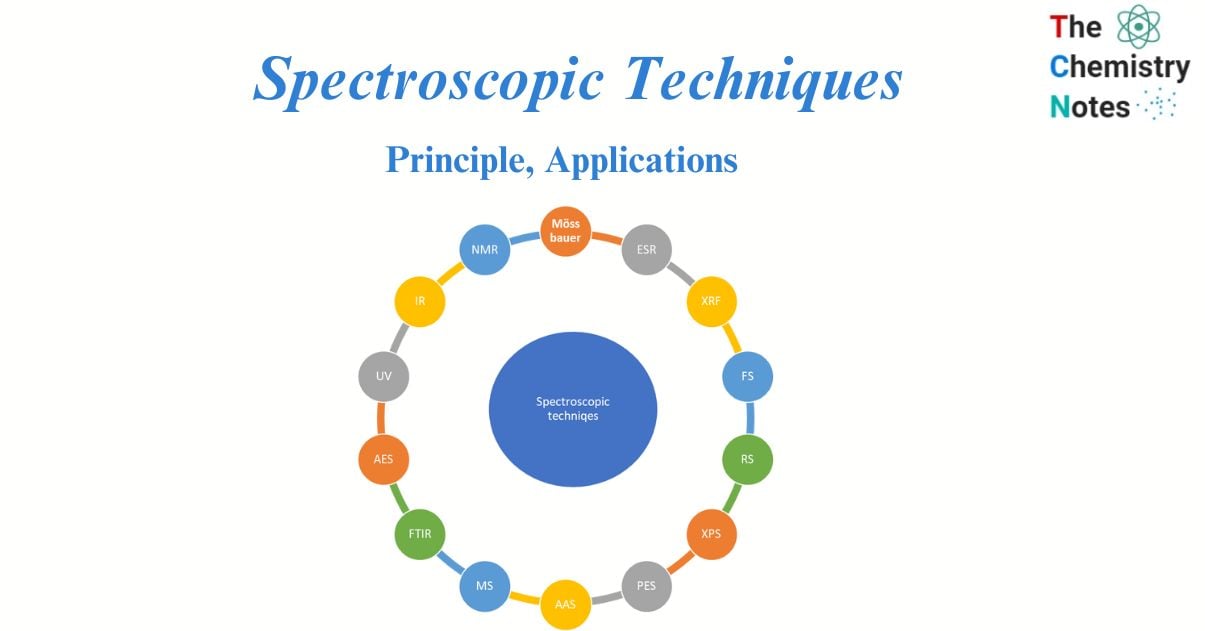
Types of Spectroscopic techniques
Nuclear magnetic resonance (NMR)
Nuclear magnetic resonance (NMR) spectroscopy is a physicochemical technique for determining the structural properties of molecules. It is described as the study of molecules by recording radiofrequency electromagnetic radiation interactions with the nuclei of molecules placed within a strong magnetic field. NMR Spectroscopy, which is based on the nuclear magnetic resonance phenomenon, offers comprehensive information on the structure, reaction state, and chemical surroundings of molecules.
Fluorescence Spectroscopy (FS)
Fluorescence Spectroscopy is a set of techniques that deals with the measurement of fluorescence emitted by substances when exposed to ultraviolet, visible, or other electromagnetic radiation.
Raman spectroscopy (RS)
Raman spectroscopy is a type of vibrational spectroscopy that allows for the simple interpretation and very sensitive structural identification of trace amounts of substances based on their distinctive vibrational properties. Sir C.V Raman, an Indian physicist, demonstrated Raman spectroscopy, which is based on the inelastic scattering of monochromatic light with the sample. Because of the inelastic scattering, the resultant light will have a different frequency than the incident light.
This method is commonly used to investigate vibrational, rotational, and other low-frequency interactions in molecules. This is quite beneficial for identifying molecular structure, identifying functional groups, etc.
Fourier Transform Infrared (FTIR) spectroscopy
Fourier Transform Infrared (FTIR) spectroscopy is the preferred method of infrared spectroscopy. IR radiation is transmitted through a sample in infrared spectroscopy. The sample absorbs some of the infrared energy, and some of it is transferred (passed through). The resulting spectrum gives the sample a molecular fingerprint by displaying the molecule’s absorption and transmission.
X-ray photoelectron spectroscopy (XPS)
X-ray photoelectron spectroscopy is a surface-sensitive analytical technique that involves bombarding a material’s surface with X-rays and measuring the kinetic energy of the released electrons. The surface sensitivity and capacity to reveal chemical state information from the elements in the sample are two significant properties of this approach that make it powerful as an analytical tool.
Auger electron spectroscopy
Auger electron spectroscopy (AES) is a type of electron spectroscopy that detects electrons produced by Auger relaxation processes. It is a non-destructive core-level electron spectroscopy used to determine the elemental composition of surfaces, thin films, and interfaces in a semi-quantitative manner.
Atomic emission spectroscopy
Atomic emission spectroscopy (AES) is an analytical technique used to quantify metal atoms by measuring the intensity of light produced by the atoms in excited states. When an excited atom returns to its ground state, it emits a specific wavelength of radiation. Excitation (absorption of radiation) and de-excitation (emission of radiation) of electrons are both involved in atomic emission spectroscopy.
Atomic absorption spectrometry
Atomic absorption spectrometry (AAS) detects elements in liquid or solid samples by using certain wavelengths of electromagnetic radiation from a light source. Individual elements absorb wavelengths differently, and the absorbances of these elements are evaluated against standards. The basis of atomic absorption spectroscopy (AAS) is that free atoms in their ground state can absorb light of a specific wavelength.
Photoelectron spectroscopy
Photoelectron spectroscopy (PES) is an experimental method for determining atomic and molecular electron energies. Photoelectron spectroscopy primarily employs a photon to interact with an electron, while other parts of the electromagnetic spectrum can also be used for spectroscopy.
Mass spectroscopy
Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. Mass spectrometry is an analytical technique that can be used to determine the mass-to-charge ratio (m / z) of one or more molecules in a sample. These measurements are frequently used to determine the exact molecular weight of the sample components. Mass spectrometry is an analytical method for determining the molecular mass of a molecule that has indirectly contributed to the identification of isotopes.
Infrared spectroscopy
Infrared spectroscopy is the measurement of the interaction of IR radiation with compounds. It is based on the principle that When IR radiation is passed through the IR active compounds, they will get excited and show specific vibrational rotational spectra, which are characteristic of the functional group of compounds.
The X-ray fluorescence (XRF)
The X-ray fluorescence (XRF) spectrometer is an analytical instrument that employs X-ray technology to perform routine and minimally invasive chemical analyses of various geological materials such as rocks, minerals, sediments, and fluids. The operational principles of this system are based on wavelength-dispersive spectroscopy.
UV spectroscopy
UV spectroscopy is an analytical technique that compares the amount of discrete wavelengths of UV or visible light absorbed or transmitted by a sample to a reference or blank sample. The sample composition influences this attribute, which may provide information about the nature and concentration of the sample. UV-Vis spectroscopy is a widely used technique in many fields of science, including bacterial culture, drug identification, and nucleic acid purity checks and quantification, as well as quality control in the beverage industry and chemical research.
Spin resonance spectroscopy
It is the study of energy levels associated with electron or nuclear spin orientations using a magnetic field. Nuclear magnetic resonance (NMR) is a radio-frequency method, whereas electron spin resonance (ESR) uses microwave radiation absorption.
Circular Dichroism Spectroscopy
Circular Dichroism Spectroscopy involves measuring the difference in absorption levels between two big signals. As a result, the approach is highly vulnerable to noise, and measurements must be performed with care. The primary application of protein CD spectroscopy is secondary structure verification. The use of CD to determine tertiary structure is limited due to a lack of theoretical knowledge of the impacts of different components of molecules at this level of structure.
Mössbauer spectroscopy
Mössbauer spectroscopy includes the resonant absorption of a γ-ray photon by a nucleus. The resonant state is achieved by changing the velocity of a sample relative to the source using the Doppler effect. The chemical environment of the nucleus generates distinct frequency changes.
Applications of Spectroscopic techniques
- Spectroscopic techniques are mostly used to investigate the structure of molecules and atoms. Spectroscopy will explore the structure and electron configurations of atoms and molecules using a long wavelength.
- Spectroscopic techniques can also be used to determine the chemical makeup of unknown materials. The emission spectrum of spectroscopy will aid in identifying a few parts per million of a trace element in a substance.
- Spectroscopic techniques are used to examine the atmospheres of cool stars for molecular species, molecular clouds where new stars emerge, and the Doppler shifts of galaxies to trace their movements.
- Spectroscopic techniques aid in biochemical studies such as DNA melting, proteins, enzymes, nucleic acids, and so on, as well as surface characterization, chemical kinetic studies on reactants and products of reactions, qualitative and quantitative analysis of transition metals, and studies of chemical and biochemical processes, and isotopic studies.
- The analysis of air pollutants, organic and inorganic contamination in wastewaters, and natural water bodies is made easier by spectroscopic techniques. The analysis of air pollutants, organic and inorganic contamination in wastewaters, and natural water bodies is made easier by spectroscopic techniques.
- Spectroscopic techniques aid in the improvement of different qualities such as heating, irradiation, and impregnating gems with oils, waxes, and so on.
References
- https://www.britannica.com/science/spectroscopy
- https://andor.oxinst.com/learning/view/article/fundamentals-of-spectroscopy-history-explanations-and-applications
- https://www.platypustech.com/5-different-types-of-spectroscopy
- https://research-repository.griffith.edu.au/bitstream/handle/10072/34561/62679_1.pdf
- https://chemicalnote.com/spectroscopy-an-overview-introduction-types-applications/
- https://www.vedantu.com/physics/spectroscopy
- https://www.pasco.com/products/guides/what-is-spectroscopy
