Interesting Science Videos
Acidic Amino Acids Definition
Acidic amino acids are those amino acids that have a carboxylic acid group on their side chains at neutral pH, resulting in acidic properties in the molecule.
- Acidic amino acids have an additional carboxylic group (COOH) in addition to the one present on the linear chain.
- These types of amino acids have acidic properties and, thus, can form ionic bonds or salt bridges with different molecules.
- There are two amino acids that belong to this group; aspartic acid and glutamic acid.
- The aqueous solution of these amino acids at physiological pH results in the ionization of the three functional groups present on the amino acids. This results in the overall charge of -1.
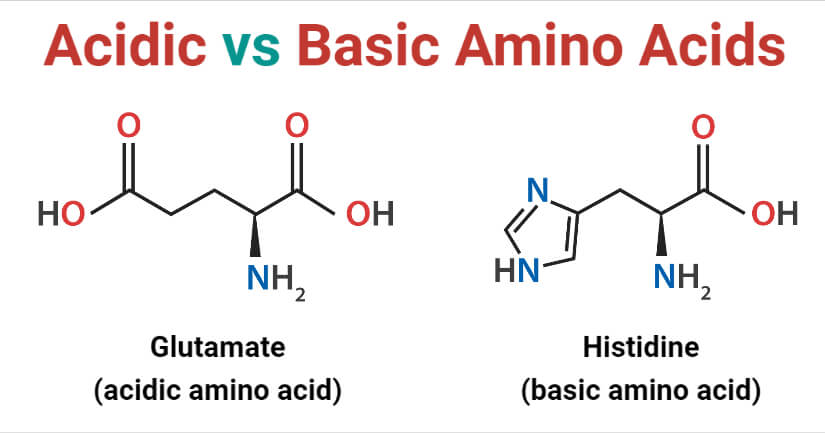
- The ionized forms of the two acidic amino acids are aspartate and glutamate. The side chains on these amino acids can form ionic bonds with other molecules.
- These amino acids occur in many proteins that bind metal ions (metalloproteins) for structural and functional purposes as the side chains of these amino acids can act as hydrogen bond acceptors.
- Glutamate is also the most abundant excitatory neurotransmitter in the central nervous system. Free glutamate is also essential in amino acid metabolism.
Basic Amino Acids Definition
Basic amino acids are those amino acids that have an amino group on their side chains at neutral pH, resulting in basic or alkaline properties in the molecule.
- Basic amino acids have an additional amino group (NH2) in addition to the one present in the central chain.
- These amino acids have basic properties as the amino group can gain protons.
- There are three amino acids that belong to this group; arginine, histidine, and lysine.
- Arginine and lysine both have an overall charge of +1 at physiological pH as a result of ionization. The Arginine side chain contains a guanidine group which is the most basic of all R groups found in amino acids.
- The side chain in histidine is an imidazole side chain that allows it to function in both acid and base catalysis at physiological pH values. Histidine is thus, often used to make up the active sites of protein enzymes.
- Basic amino acids are hydrophilic and thus, are found clustered on the surface of globular proteins in solutions.
7 Major Differences (Acidic Amino Acids vs Basic Amino Acids)
| Characteristics | Acidic Amino Acids | Basic Amino Acids |
| Definition | Acidic amino acids are those amino acids that have a carboxylic acid group on their side chains at neutral pH, resulting in acidic properties in the molecule. | Basic amino acids are those amino acids that have an amino group on their side chains at neutral pH, resulting in basic or alkaline properties in the molecule. |
| Side Chain | The side chain in acidic amino acids is acidic. | The side chain in basic amino acids is basic. |
| Properties | Acidic amino acids contain a carboxylic acid side chain which results in acidic properties in the molecule. | Basic amino acids contain an amino group in the side chains, which results in basic properties in the molecule. |
| pKa value | The pKa values of acidic amino acids are low. | The pKa values of basic amino acids are high. |
| Ionization | Acidic amino acids form anions as they can lose protons. | Basic amino acids form cations as they gain protons. |
| Charges | Acidic amino acids have a negative charge in their side chain. | Basic amino acids have a positive charge in their side chain. |
| Examples | Examples of acidic amino acids are aspartic acid and glutamic acid. | Examples of basic amino acids are histidine, lysine, and arginine. |
Examples of acidic amino acids
1. Glutamic acid
- Glutamic acid is an acidic amino acid that is essential for the biosynthesis of different kinds of proteins in living beings.
- Glutamic acid is one of the non-essential amino acids that can be synthesized in the body, but it is the most abundant excitatory neurotransmitter in the vertebrate nervous system.
- The molecular formula of glutamic acid is C5H9O4N with the molecular structure COOH-CH(NH2)-(CH2)2-COOH. The amino acid consists of two carboxylic acid groups and a single amino group.
- The amino acid can lose a proton from the carboxylic group present on the side chain to form a glutamate anion.
- Glutamic acid is involved in the biosynthesis of proteins, and it can be tasted when present in an unbound form.
- Common foods that contain free glutamic acid include cheeses and soy sauce, where glutamic acid adds the umami flavor to these foods.
2. Aspartic acid
- Aspartic acid is the other acidic amino acid found in the living system, which is also a non-essential amino acid in humans.
- The molecular formula of aspartic acid is C4H7NO4 with the molecular structure COOH-CH2-CH(NH2)-COOH.
- Aspartic acid is essential for the synthesis of other amino acids that play essential roles in processes like urea and citric acid cycles.
- Amino acids like lysine, methionine, isoleucine, and arginine are synthesized from aspartic acid.
- Aspartic acid consists of an additional carboxylic acid that can release a protein and acquire a negative charge at the physiological pH of body fluids.
- Aspartic acid is often designated by the name of its ionized form, aspartate. The IUPAC name of the amino acid is 2-aminobueanedioic acid.
- The amino acid can exist in two enantiomeric forms, L-aspartate and D-aspartate, where L-aspartate is essential for protein biosynthesis and neurotransmission.
Examples of basic amino acids
1. Histidine
- Histidine is a basic amino acid that is considered semi-essential as children require to obtain it from food, consisting of an additional amino group.
- The molecular formula of histidine is C6H9N3O2 with an imidazole side chain that remains positively charged at physiological pH.
- Histidine is an essential amino acid that is a precursor to histamine, a viral inflammatory agent involved in immune responses.
- The imidazole side chain of the amino acid has a pKa value of around 6.0, below which the ring remains protonated. At a pH above 6, the ring loses a proton resulting in a negatively charged ion.
- The acid-base character of the side chain is useful in the catalytic activity of different enzymes.
- Histidine also forms complexes with many metal ions acting as a ligand in metalloproteins.
2. Lysine
- Lysine is an essential amino acid in humans, which cannot be synthesized within the body by human beings and thus has to be supplied through foods.
- It is a basic amino acid with a side chain lysyl ((CH2)4NH2) with the molecular formula of C6H14N2O2.
- Lysine plays an important role in proteogenesis and in the crosslinking of collagen polypeptides and uptake of mineral nutrients.
- The lack of lysine can lead to several diseases like defective connective tissues, impaired fatty acid metabolism, and protein-energy deficiency.
- Lysine exists in two enantiomeric forms, D-lysine and L-lysine, and the L-isomer is found in humans.
References and Sources
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 5962, Lysine” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Lysine. Accessed 20 February, 2021.
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 5962, Lysine” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Lysine. Accessed 20 February, 2021.
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 5962, Lysine” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Lysine. Accessed 20 February, 2021.
- 3% – https://www.wikidoc.org/index.php/Amino_acids
- 1% – https://www2.chem.wisc.edu/deptfiles/genchem/netorial/modules/biomolecules/modules/protein1/prot14.htm
- 1% – https://www.differencebetween.com/difference-between-acidic-and-vs-basic-amino-acids/
- 1% – https://www.chegg.com/homework-help/arginine-contains-guanidine-group-side-chain-basic-20-common-chapter-15-problem-40ap-solution-9780534352158-exc
- 1% – https://www.britannica.com/science/amino-acid/Standard-amino-acids
- 1% – https://www.assignmentpoint.com/science/chemistry/glutamic-acid-a-non-essential-amino-acid.html
- 1% – https://pediaa.com/what-is-the-difference-between-lysine-and-l-lysine/
- 1% – https://pediaa.com/what-is-the-difference-between-acidic-and-basic-amino-acids/
- 1% – https://en.wikipedia.org/wiki/Lysyl
- 1% – https://en.wikipedia.org/wiki/Glutamates
- 1% – https://en.wikipedia.org/wiki/Glutamate_(neurotransmitter)
- 1% – https://byjus.com/chemistry/histidine/
- <1% – https://www.researchgate.net/publication/51177251_To_Give_or_Not_to_Give_Lessons_from_the_Arginine_Paradox
- <1% – https://www.khanacademy.org/test-prep/mcat/biomolecules/amino-acids-and-proteins1/v/classification-amino-acids
- <1% – https://www.healthline.com/health/erectile-dysfunction/l-lysine-deficiency-and-ed
- <1% – https://www.coursehero.com/tutors-problems/Psychology/24652589-The-side-chain-imidazole-ring-of-histidine-has-a-pKa-60-therefore/
- <1% – https://wikimili.com/en/Glutamic_acid
- <1% – https://quizlet.com/102134698/1-amino-acids-peptides-and-proteins-flash-cards/
- <1% – https://in.answers.yahoo.com/question/index?qid=20111004090412AALdggy
- <1% – https://en.wikipedia.org/wiki/Non-proteinogenic_amino_acids
- <1% – https://en.wikipedia.org/wiki/L-histidine
- <1% – https://en.wikipedia.org/wiki/Amino_acid
- <1% – https://aminoco.com/blogs/amino-acids/acidic-and-basic-amino-acids
- <1% – http://www.medicinebau.com/uploads/7/9/0/4/79048958/1-_amino_acids.pdf
