Adsorption is a surface phenomenon that involves the accumulation of molecular species on the surface rather than within the bulk of a solid or liquid.
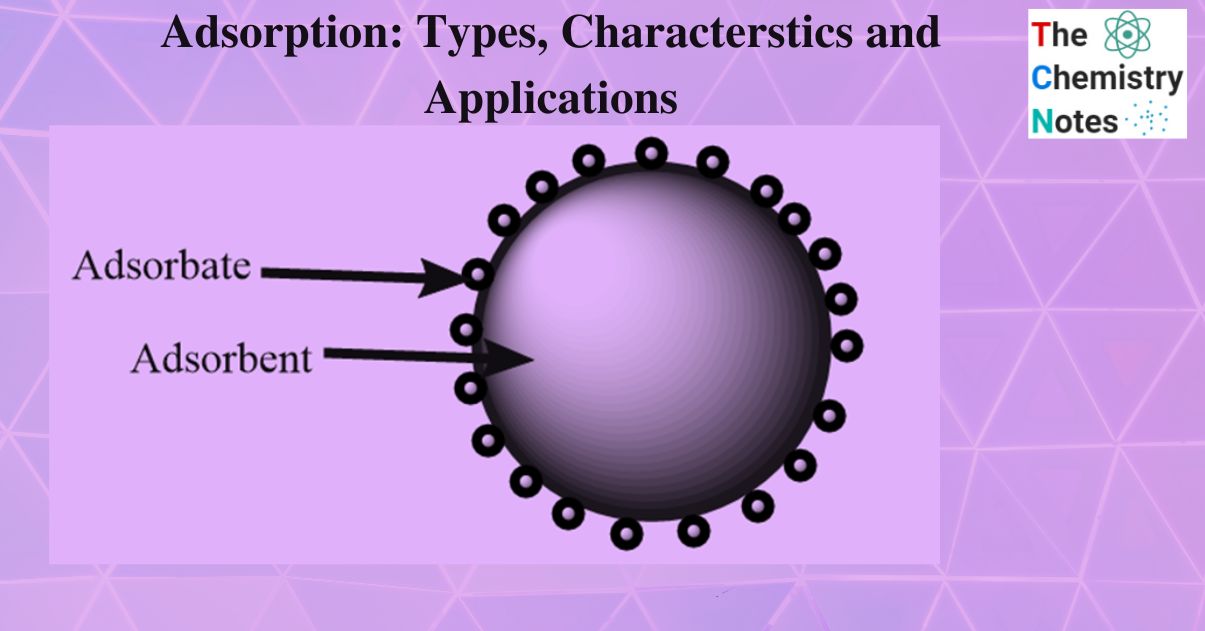
A substance that accumulates on the surface of another substance is called adsorbate. For instance, H2, N2, and O2 gases. The adsorbent is the material on the surface on which adsorption occurs. For example, Charcoal, Silica gel.
Solids with a large surface area, particularly those in finely divided form, such as charcoal, silica gel, alumina gel, finely divided metals, and so on, act as good adsorbents.
Interesting Science Videos
Mechanism of adsorption
The adsorbent’s forces are responsible for attracting adsorbate particles to its surface. The extent of adsorption increases with increasing surface area per unit mass of adsorbent at a given temperature and pressure. The residual forces of the surface always decrease during adsorption. It is an exothermic process. The ∆H of adsorption is always negative. Due to adsorption of gases, the molecules’ freedom of movement is restricted. This corresponds to a decrease in the entropy of the gas following adsorption, i.e., ∆S is negative.
According to the equation, ∆G = ∆H – T∆S, ∆G must be negative for a process to be spontaneous. AS adsorption is a spontaneous process, in which the combination of ∆H and – T∆S results in ∆G negative.
Adsorption in action
- On addition of animal charcoal to a solution of an organic dye, say methylene blue,the filtrate becomes colorless. As a result, the dye molecules adsorbed on the surface of the charcoal.
- An aqueous solution of raw sugar on passing over beds of animal charcoal,the solution becomes colorless due to adsorption of coloring agent.
- In presence of silica gel the air becomes dry. This is due to adsorbtion of water molecules on the surface of silica gel.
Types
Based on the interaction forces between adsorbate and adsorbent, there are two types of adsorptions. They are:
Physical adsorption or Physisorption
The reversible phenomenon that involves the weak Van der Waals forces between adsorbate and adsorbent. E.g., the Adsorption of H2 on the surface of the charcoal.
Characteristics
Not specific
It is non-specific and occurs in all types of adsorbents.
Nature of gas
As the Vander Waals’ forces are stronger near the critical temperatures., easily liquefiable gases (i.e., with higher critical temperatures) are readily adsorbed on the surface of the adsorbent.
Reversible nature
Physisorption of a gas by a solid is usually reversible. So, Solid + Gas l Gas/Solid + Heat
Physisorption of gas increase with the increase in pressure, while the gas can be removed by decreasing the pressure.
Surface of adsorbent
It increases with adsorbent surface area. As a result, finely divided metals and porous substances with large surface areas are effective adsorbents.
Enthalpy
The enthalpy of Physisorption is quite low (20–40 kJ mol-1).
Chemical adsorption or Chemisorption
It is an irreversible phenomenon that involves the strong chemical interaction between adsorbate and adsorbent. For example, Iron on heating withN2 gas at 623 K produes iron nitride on the surface .
Characteristics
Higher specificity
Chemisorption is highly specific and will only occur if chemical bonding between adsorbent and adsorbate is possible.
Irreversibility
Chemisorption is the irreversible process that involves compound formation.
The surface area of the adsorbent
Chemisorption also increases with adsorbent surface area.
Enthalpy :
The enthalpy of chemisorption is high (80-240 kJ mol-1).
Commonly used adsorbent
The adsorbent must have a large surface area. They must not lose their shape or size. Adsorbents are typically granular in size, ranging from 0.5 mm to 12 mm. The commonly used adsorbents, their source, and their applications are as follows:
| S.N. | Adsorbent | Source | Apllication |
| 1. | Activated clay | Bernolite or other activated clay is activated through sulfuric acid treatment, followed by washing, drying, and crushing. | Decolorizes petroleum products. |
| 2. | Bone – Char | Destructive distillation of crushed bone at 600-900oC produces bone char. | Used for sugar refining and after washing and burning, it can be reused |
| 3. | Activated carbon | i. Carbonization is achieved after the vegetable matter is mixed with calcium chloride, and the inorganic matter is leached out. ii. Carbonization of materials like wood, sawdust, and coconut shells, followed by activation with a hot air stream. It gives granular and pelleted forms of activated carbon. | i.. Gold and silver recovery from cyanide ore – leach solution ii. Fractionation from hydrocarbon gas Collection of iii. Hydrocarbons from natural gas |
| 4. | Silics gel | After acid treatment, a hard granular and porous product is formed from sodium silicate solution. | Dehydration of air and fractionation of hydrocarbons |
| 5. | Molecular seives | They are metal aluminosilicates and porous synthetic zeolite crystals. | Dehydration of gases and liquids and separation of gas – liquid hydrocarbon mixture |
Factors affecting Adsorption
Temperature
Adsorption is an exothermic process and at the given pressure low temperature favors this process. When the temperature is raised, the adsorbed molecules are removed from the adsorbent, a process known as desorption. It is thus inversely proportional to temperature. However, due to the high energy of activation the extent of Chemisorption increases initially and decreases as the temperature rises.
Pressure
When a gas is adsorbed on the surface of an adsorbent, the volume of the adsorbent decreases. Adsorption increases with increasing pressure at a constant temperature, according to the Le- Chatelier principle. Over a limited pressure range, adsorption is directly proportional to the pressure of the gas.
Nature of adsorbent
Adsorption increases as the adsorbent surface area increases. The finely divided or rougher the surface of the adsorbent higher will be the adsorption.
Nature of adsorbate
As the Vander Waals’ forces are stronger near the critical temperatures., easily liquefiable gases (i.e., with higher critical temperatures) are readily adsorbed on the surface of the adsorbent.
In physisorption, the extent of adsorption depends on the boiling point of gases. Gases are more easily adsorbed on solids than liquids.
Applications
Application of adsorption chromatography
In chromatographic analysis, the selective adsorption of certain substances from a solution by a specific solid adsorbent is used to separate the components of the mixture.
Production of high vacuum
The remaining traces of air can be adsorbed by charcoal from a vessel evacuated by a vacuum pump to achieve a very high vacuum.
In pharmaceutical industry
Activated charcoal adsorbs the poisonous and toxic substances like magnesium oxide, tannic acid, and other substances.
Separation of inert gases
As the degree of adsorption of gases by charcoal is different, a mixture of noble gases can be separated by adsorption at different temperatures.
In curing disease
Some drugs can adsorb germs on their surface and thus kill them.
Heterogenous catalysis
Most of the heterogeneous catalytic reactions involve the gaseous reactant adsorbing on the solid catalyst.
Gas masks
Gas masks use the adsorbent to protect against poisonous gases such as methane (CH4), chlorine (Cl2), sulfur dioxide (SO2), and others. Adsorbents preferentially adsorb poisonous gases, thereby purifying the air.
Removal of coloring matter
By adsorbing colored impurities, animal charcoal removes the colors from solutions.
Water treatment
Hard water contains dissolved calcium and magnesium salts. Ion-exchange resins are commonly used to soften hard water. These resins work based on the principles of selective adsorption from solution.
References
- Hiemenz P. C. & Rajagopalan R. (1997). Principles of colloid and surface chemistry (3rd ed. rev. and expanded Paul C. Hiemenz Raj Rajagopalan). Marcel Dekker.
- https://www.diffen.com/difference/Absorption_vs_Adsorption
- https://nitsri.ac.in/Department/Chemical%20Engineering/Adsorption.pdf
- https://byjus.com/jee/adsorption/
- https://www.britannica.com/science/adsorption
- https://www.embibe.com/exams/application-of-adsorption/
- https://thefactfactor.com/facts/pure_science/chemistry/physical-chemistry/factors-affecting-adsorption/11184/
- https://www.toppr.com/guides/chemistry/surface-chemistry/adsorption/
- http://aevnmont.free.fr/SACH-BOOKS/Adsorption/Ruthven-Adsorption.pdf
