Americium, which belongs to the actinide class of the Periodic Table, has an atomic number of 95. It is represented using the symbol ‘Am’. It is transuranic radio element with silvery shiny appearance. It is an element that has been synthesized. Similar to other elements in the actinide class, americium exhibits significant radioactivity. It is probably found naturally on Earth, although only in trace amounts in uranium minerals, where nuclear processes may infrequently yield an atom. It is located on the very last row of the periodic table, underneath europium, and earned its name as due to of its link with another element named after a continent. Glenn Seaborg, Leon Morgan, Ralph James, and Albert Ghiorso identified the element Americium.
Interesting Science Videos
History of Americium
- Americium was discovered in 1944 as a byproduct to military experiments through World War II (1939-1945).
- The American scientists Glenn T. Seaborg, Ralph A. James, Leon O. Morgan, and Albert Ghiorso made the discovery of Americium in 1944.
- By subjecting plutonium-239, an isotope of plutonium, to high energy neutron bombardment, they created americium. As a result, plutonium-240 was created and then subjected to a neutron bombardment.
- Following its conversion from plutonium-240 to plutonium-241, the latter underwent beta decay to become americium-241.
- This research was conducted at the University of Chicago’s Metallurgical Laboratory, currently known as Argonne National Laboratory.
- It isolation was experienced by B.B. Cunningham in the autumn of 1945. The isolated component containing Am(OH)3 is described using the isotope 241Am.
- The element name americium refers to America. Americium is found right below the lanthanide element europium, which is named after Europe.
Occurrence of Americium
- Americium is probably found naturally on Earth, although only in trace amounts in uranium minerals, where nuclear processes may rarely yield an atom.
- Although americium has no natural sources, it was most likely present at some point in the past when uranium concentrations in the area were sufficient to cause nuclear reactions.
- Americium is a synthetic element formed from other elements such as plutonium. It is frequently produced as a byproduct in nuclear power plants and nuclear weapons testing.
- There are 14 isotopes of americium with mass numbers ranging from 232 to 247 that have known half-lives. All isotopes are synthesized.
Elemental Properties of Americium
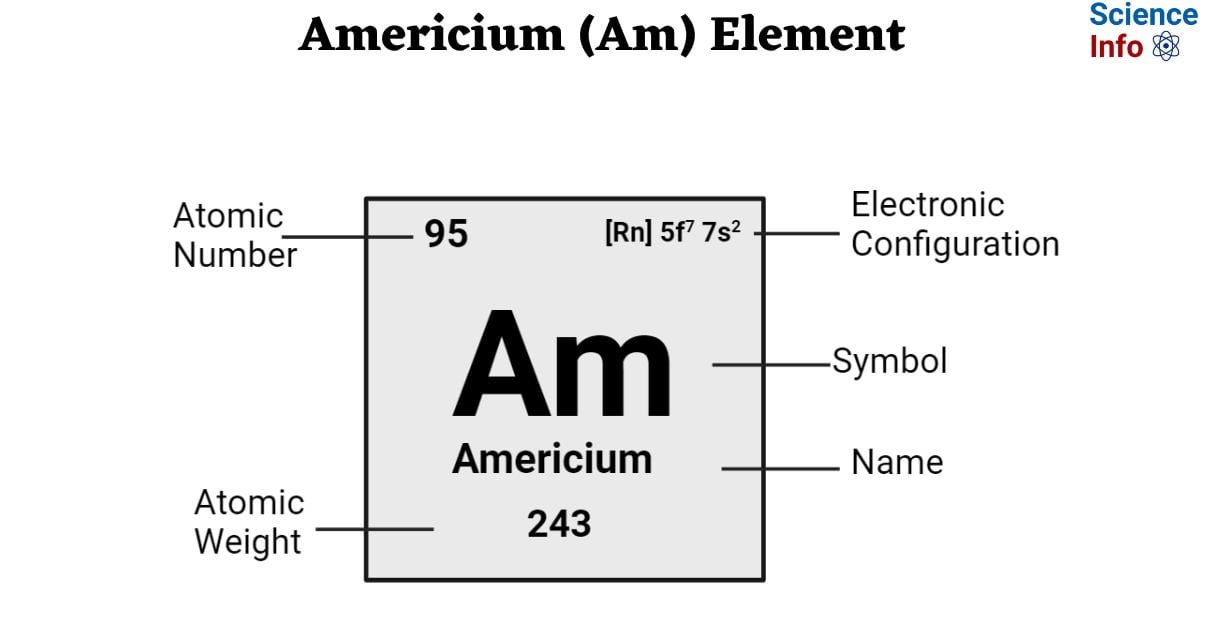
| Electronic Configuration | [Rn] 5f7 7s2 |
| Atomic Number | 95 |
| Atomic Weight | 243 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | 13.67 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 25, 8, 2 |
| Electrons | 95 |
| Protons | 95 |
| Neutrons | 148 (Varies with isotopes) |
Physical Properties of Americium
- Americium is a silvery, ductile, highly malleable, and non-magnetic metal.
- It is paramagnetic across a large temperature range, from very cold to above room temperature.
- The melting point of americium is 1,176°C, while its boiling point is 2,011°C.
- Americium has an atomic mass of 243.
- At 20°C, the density of americium is 13670 S.I. units.
- It is less dense than plutonium but denser than europium.
- Americium’s crystal structure varies depending on both pressure and temperature.
- Under typical circumstances, the metal is observed in a stable alpha form with hexagonal crystal symmetry.
- When the metal undergoes compression, it transitions to the beta form, which exhibits face-centered cubic symmetry.
- Americium changes into its gamma form, which is orthorhombic, when the pressure is increased further (23 GPa).
- Additionally, a monoclinic crystal phase has been witnessed, although its specific reasons are unknown.
- Americium, like other actinides, causes self-damage to its crystal structure via alpha decay. This is most apparent at low temperatures.
Chemical Properties of Americium
- It tarnishes gradually in dry air at room temperature.
- Americium metal reacts quickly with oxygen.
- There are five confirmed oxidation states for ammonium: +2, +3, +4, +5, and +6. The oxidation states are the most diverse of any actinide element.
- For element americium, +3 is the most prevalent oxidation state.
- The +2 oxidation state can only occur in solid substances and is highly unstable.
- The longevity of americium oxidation states greater than +3 is lower than that of uranium, neptunium, and Plutonium.
- In an aqueous solution, the ions take on color.
- The +3 state is colorless to reddish yellow, whereas the +4 state is reddish yellow, with brown and green representing the remaining states.
- It dissolves readily in aqueous solutions.
- It is known to produce halides, pnictides, chalcogenides, silicides, borides, and organo americium compounds.
Uses of Americium
- Americium-241 (241Am) is used in extremely minute quantities in residential ionization smoke detectors. Americium is comparable to plutonium (Pu) in numerous aspects. While the general population accepts the use of trace amounts of 241Am in smoke detectors in their homes, their response to conveying any amount of 239Pu under reasonable circumstances is considerably different.
- Americium-241 has been employed as a portable source of gamma rays and alpha particles for a variety of medicinal and industrial applications. It can be utilized to do indirect material analysis in radiography and X-ray fluorescence spectroscopy, as well as quality control in fixed nuclear density gauges and nuclear densimeters. For example, the element has been used to measure glass thickness in order to help make flat glass. However, producing this isotope in useable amounts is highly expensive.
- Investigation has been performed on several Americium alloys for use as a radiochemical diagnostic tracer. They have attempted to combine Americium with uranium, aluminum, and cerium. The cerium-americium alloys were the most successfully made and had the highest density, meeting the project’s objectives. However, there is little additional research or use for Americium alloys.
- It contributes to nuclear power production as a decay product.
- Due to the shortage of Plutonium for spacecraft batteries, Americium may serve as a suitable substitute in the coming years.
- Americium-241 is a useful laboratory source of alpha particles.
Toxicity of Americium
- Americium is known to be harmful due to high radioactivity, and exposure to this radiation can trigger genetic alterations in human cells, leading to bone malignancies. Radiation from americium can harm the lungs, liver, thyroid, and kidneys.
- Americium emits alpha particles, posing a substantial risk when ingested or inhaled. Once in the body, americium concentrates largely in the skeleton, liver, and muscles. It can linger in the body for decades, exposing nearby tissues to radiation. Exposure to this substance may raise the risk of developing cancer, but the consequences may take years to manifest.
- Direct external exposure to gamma ray emissions can also pose a concern with Americium.
Environmental Effects of Americium
- Americium from atmospheric nuclear weapons testing may stay in the atmosphere for decades, traveling all over the planet and gradually settling to Earth.
- Its isotopes degrade slowly in the environment, which can be harmful to plants and animals.
- Animals exposed to high quantities of americium may have organ damage, including the lungs, liver, and thyroid.
- Americium found in soils may wind up in plants, but only in trace amounts.
- Usually, americium particles are stored in plant sections that animals will not consume.
- Americium accumulates barely in flesh of fish or other edible portions, therefore it does not accumulate in food chains.
Video Reference
References
- https://www.atsdr.cdc.gov/toxprofiles/tp156-c4.pdf
- https://www.lenntech.com/periodic/elements/am.htm
- https://www.thoughtco.com/americium-facts-element-95-4124371
- https://www.britannica.com/science/americium
- https://byjus.com/chemistry/americium/
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
Tables of Physical & Chemical Constants, Kaye & Laby Online, 16th edition, 1995. Version 1.0 (2005), accessed December 2014. - https://www.chemicool.com/elements/americium.html
- https://chemistrydictionary.org/americium/
- https://chemicalengineeringworld.com/americum-element-properties-and-information/
- https://www.chemistrylearner.com/americium.html


… “While the general population accepts the use of trace amounts of 241Am in smoke detectors in their homes… ”
Uh, not this member of the general pop. If it was my call, the use of this extremely hazardous radioactive shit in these devices would’ve been BANNED years ago…