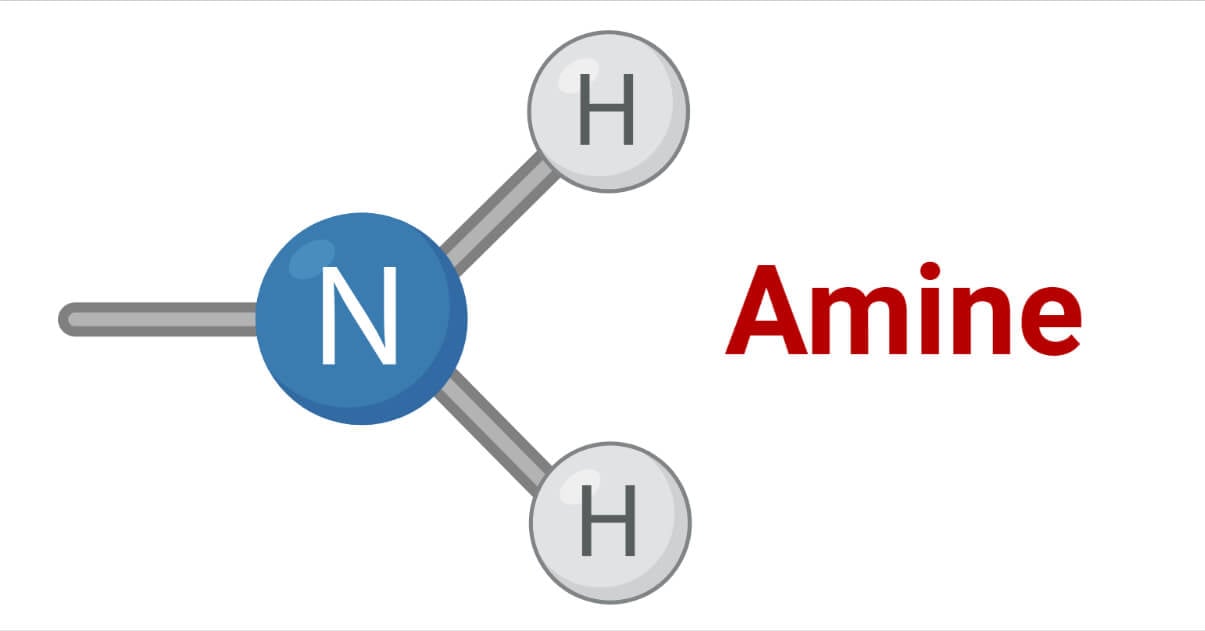
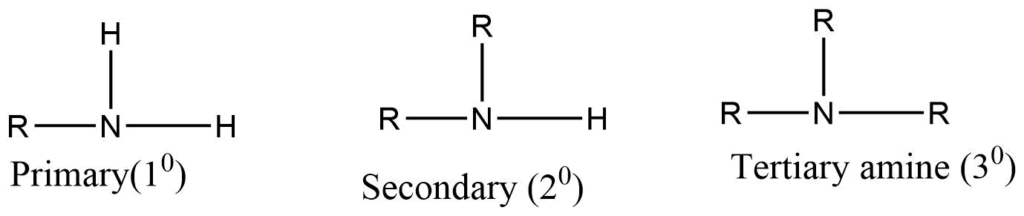
Amines are the class of organic compounds obtained by replacing one, or more hydrogen atoms of ammonia with alkyl groups. On the basis of the number of alkyl groups present in the compounds, amines are classified as primary secondary, tertiary.
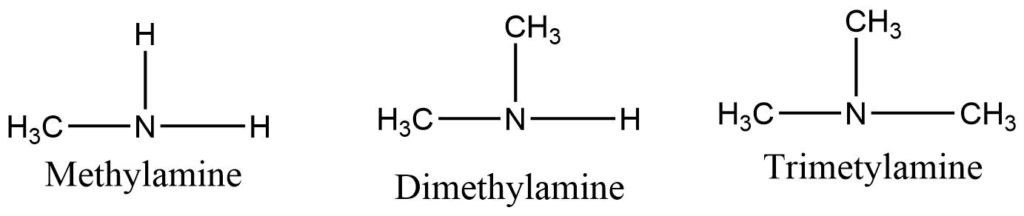
Primary amine: Amines containing only one alkyl group attached to the nitrogen. E.g. Methyl amine
Secondary amine: Secondary amine consists of two alkyl groups directly attached to the nitrogen atom. E.g. Dimethyl amine
Tertiary amine: Tertiary amine has three alkyl groups directly attached to nitrogen. E,g Trimethyl amine.
Cyclic amines: They are the amines in which the secondary and tertiary amines are present in cyclic form. E.g: Aziridine, Pyrrolidine, Piperidine

Interesting Science Videos
Structure of Amines
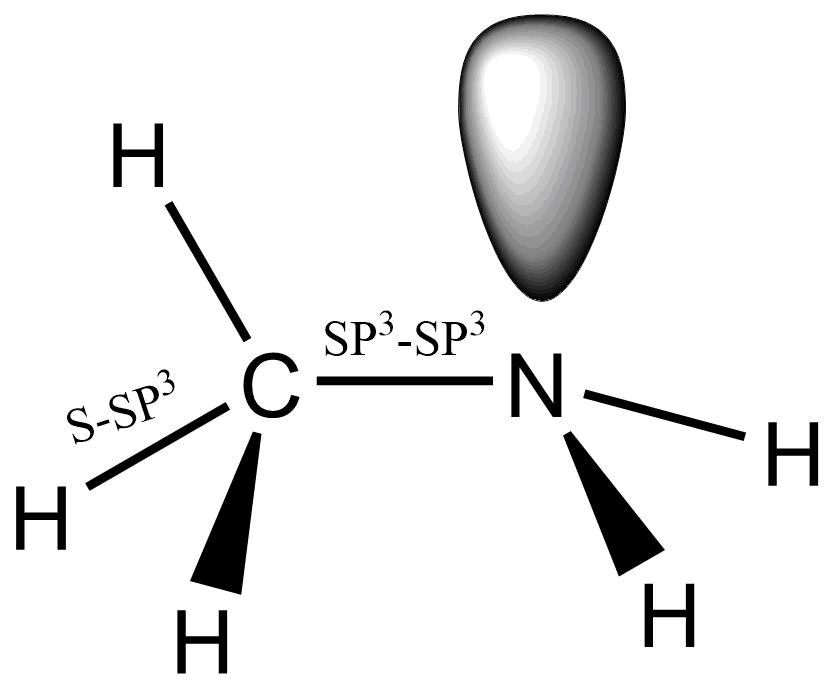
The nitrogen in amines is SP3 hybridized. Due to the presence of lone pairs of electrons, it has a pyramidal structure rather than a tetrahedral. Depending on the nature of amine,the SP3 hybridized orbital of nitrogen overlap with the orbital of hydrogen and carbon. The C-N-H bond angle of amines is 107 degrees, which is slightly different from that of tetrahedral bonds due to the presence of a lone pair of electrons.
Nomenclature of Amines
a. Amines are named by naming the alkyl group attached to the nitrogen atom followed by the ending -amine.
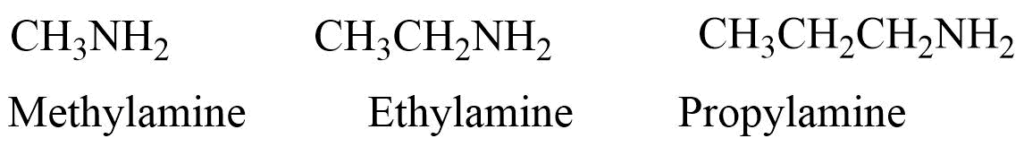
B. If two or more same alkyl groups are present then the prefix di-, or tri is used.

c. If two or more different alkyl groups are present then they are named on the basis of alphabetical order.
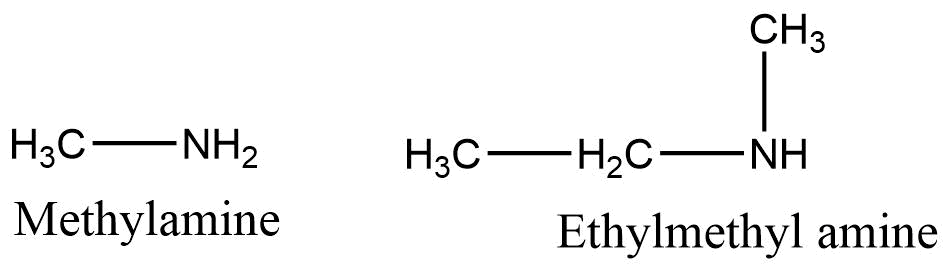
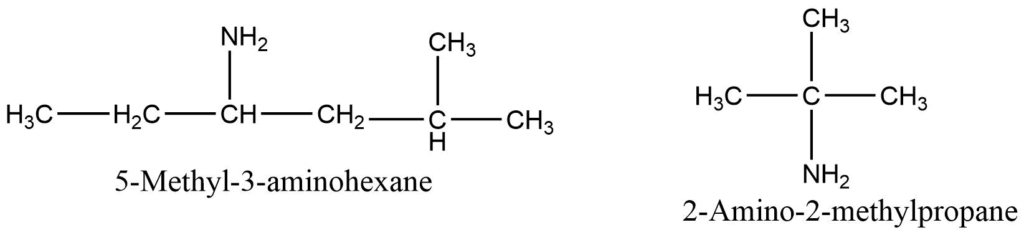
d. The complex animes are generally named according to the IUPAC name. Where amino group (-NH2) is considered as the functional group and denoted by the lowest possible number.
Methods of preparation of Amines
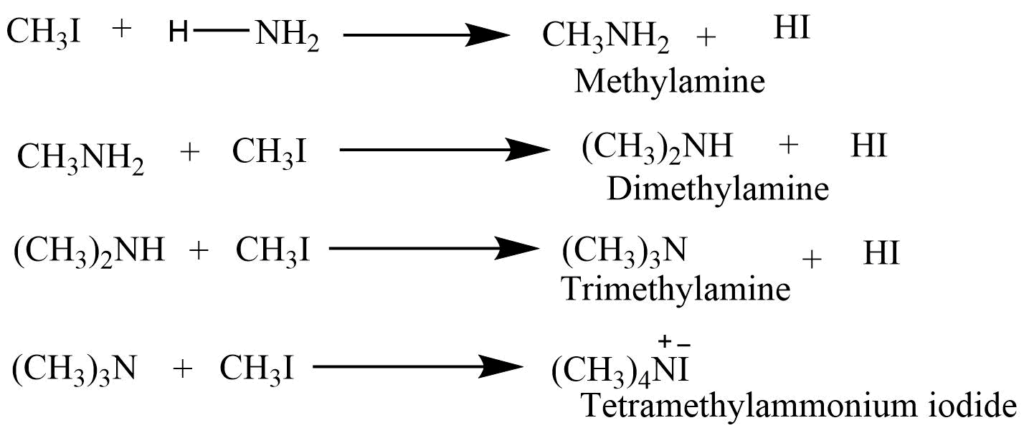
Reaction of an alkyl halide with ammonia
The reaction of an alkyl halide with ammonia in a sealed tube form the mixture of the primary, secondary, tertiary, and quaternary ammonium salt. As this reaction leads to the formation of the mixture of ammonium salt it is not suitable for laboratory synthesis.
Reaction of alcohols with ammonia
On passing the vapors of alcohol and ammonia over heated alumina at 4000C, the mixture of the primary secondary, and tertiary amine is produced.
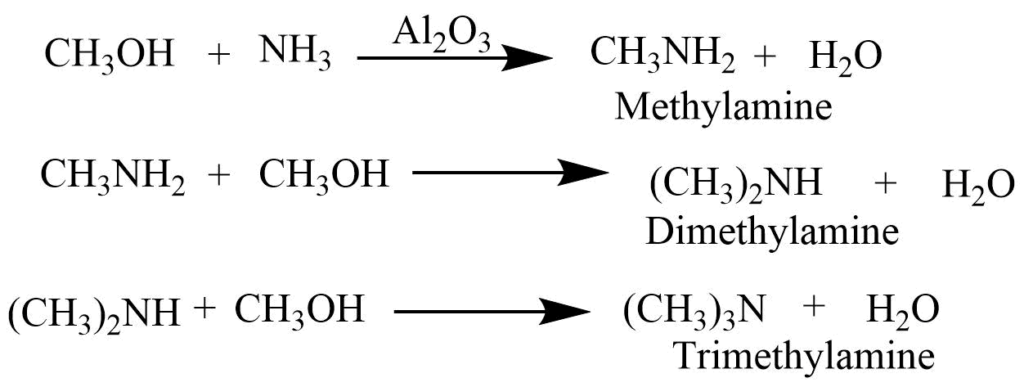
Preparation of primary amines
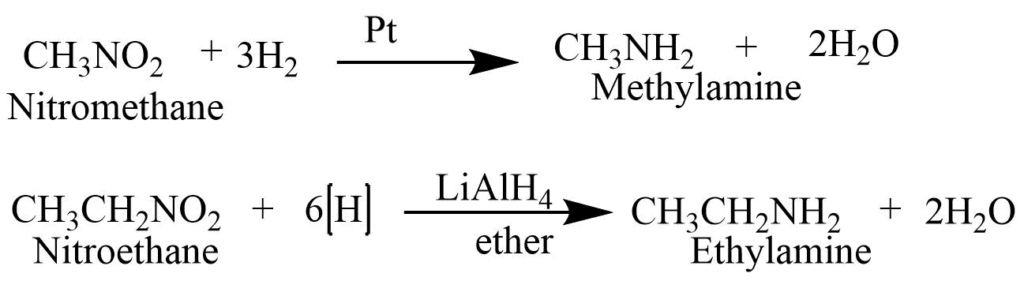
Reduction of nitroalkanes
Nitroalkane on reduction with H2/Pt or lithium aluminum hydride produces primary alkanes.
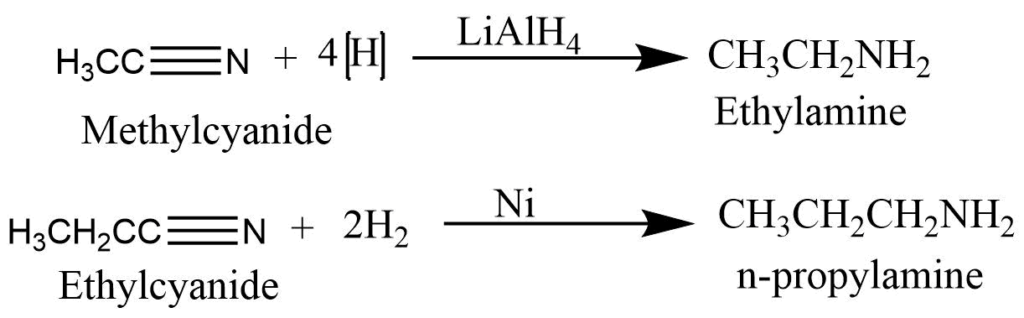
Reduction of nitriles
Reduction of nitriles (alkyl cyanide) with H2/Ni or lithium aluminum hydride produces primary amine.
From amide
Simple amide undergoes a reduction reaction with lithium aluminum hydride to form primary amine.
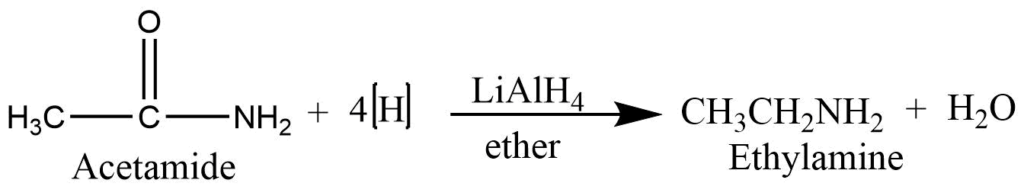
Hofmann’s Degradation of amide
Warming of the amide with bromine and the concentrated aqueous solution of NaOH solution form primary amine. It is a good method for the laboratory preparation of primary amine. This reaction is also called Hofmann’s rearrangement reaction. The product contains one carbon less than the original amide.

Methods for the preparation of secondary amines
The reaction of a primary amine with alkyl halide
Primary amine on reaction with the alkyl halide produces the dialkylammonium salt which on further reaction with NaOH yields free secondary amine.
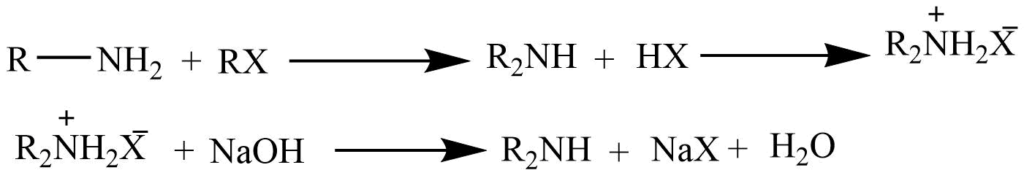
Reduction of the N-substituted amides
N-substituted amides on reduction with lithium aluminum hydride produces secondary amines.
Reduction of isonitriles: Reduction of the isonitrile or isocyanides with H2/Ni can produce secondary amine. Thus produced secondary amines will always have one CH3 group.
Method of preparation of tertiary amines:
The reaction of an alkyl halide with ammonia
Alcoholic ammonia solution on heating with an excess of alkyl halide, a tetraalkylammonium halide is formed. Which on reaction with NaOH solution gives free tertiary amine.
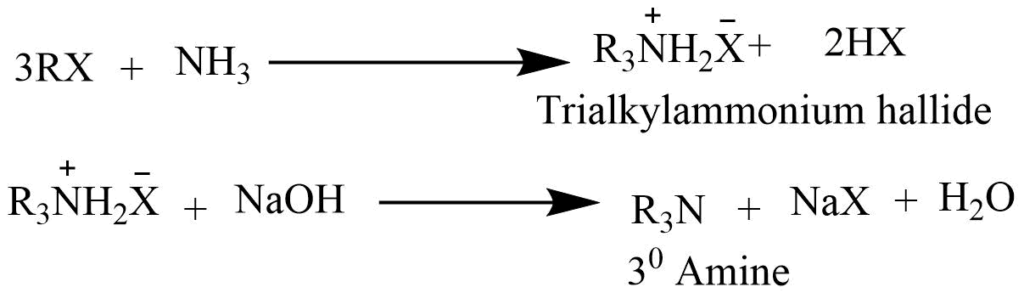
Reduction of N-N’ disubstituted amides
Reduction of N-N’ disubstituted amides with lithium aluminum hydride produces tertiary amines.
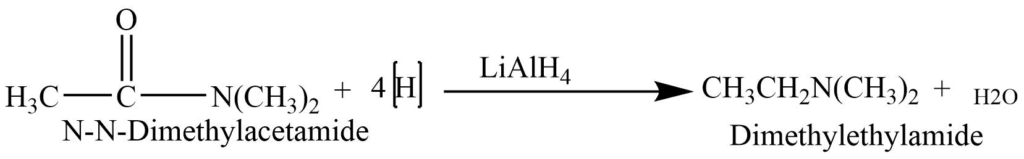
Decomposition of tetraalkylammonium hydroxide
Tetraalkylammonium hydroxide on strongly heating produces tertiary amines.

Separation of a mixture of amines
A mixture containing primary, secondary, and tertiary amines along with quaternary salt on distillation with KOH solution primary, secondary, and tertiary amines distills off. But the quaternary salt doesn’t react with KOH and being less volatile it is left behind.
The distillate containing a mixture of primary, secondary, and tertiary amines can be separated by different ways.
Fractional distillation:
A mixture of primary, secondary, and tertiary amines can be separated by fractional distillation due to differences in their boiling point.
Hofmann’s method:
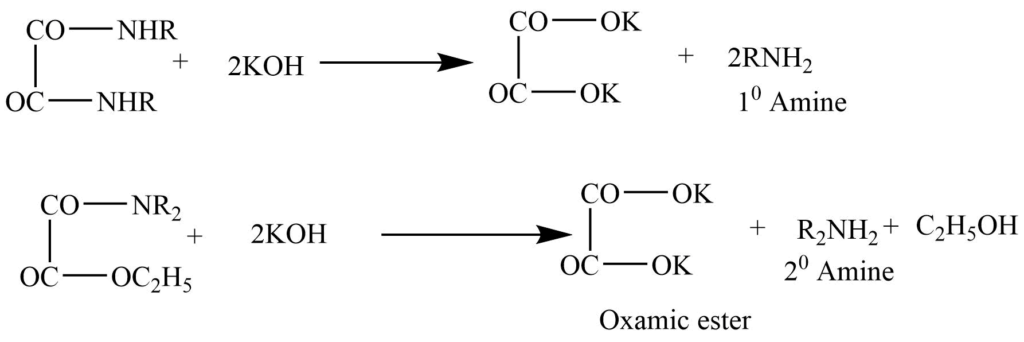
It involves the reaction of a mixture with diethyl oxalate.
a. Primary amine produces dialkyloxamide. Which is solid.
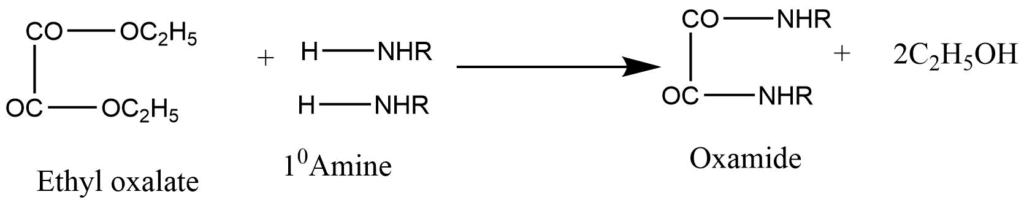
b. Secondary amines produce dialkyloxamic ester, which is an oily liquid.
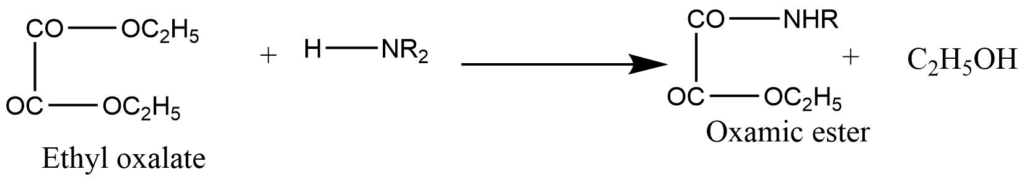
c. The tertiary amine doesn’t react with the diethyl oxalate.
In this process, the tertiary amine is first distilled over, and then the oxamic ester is obtained as the second fraction, while the primary amine remains behind in the distillation flask.
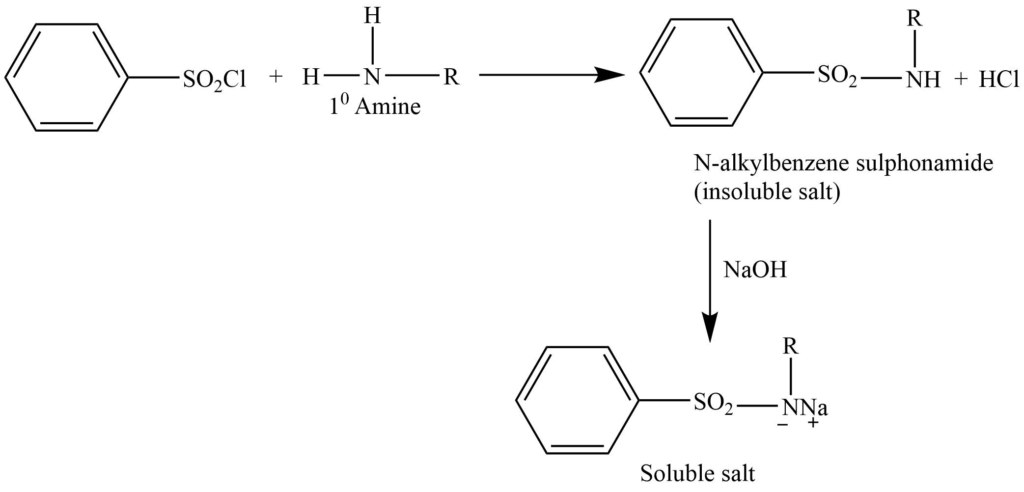
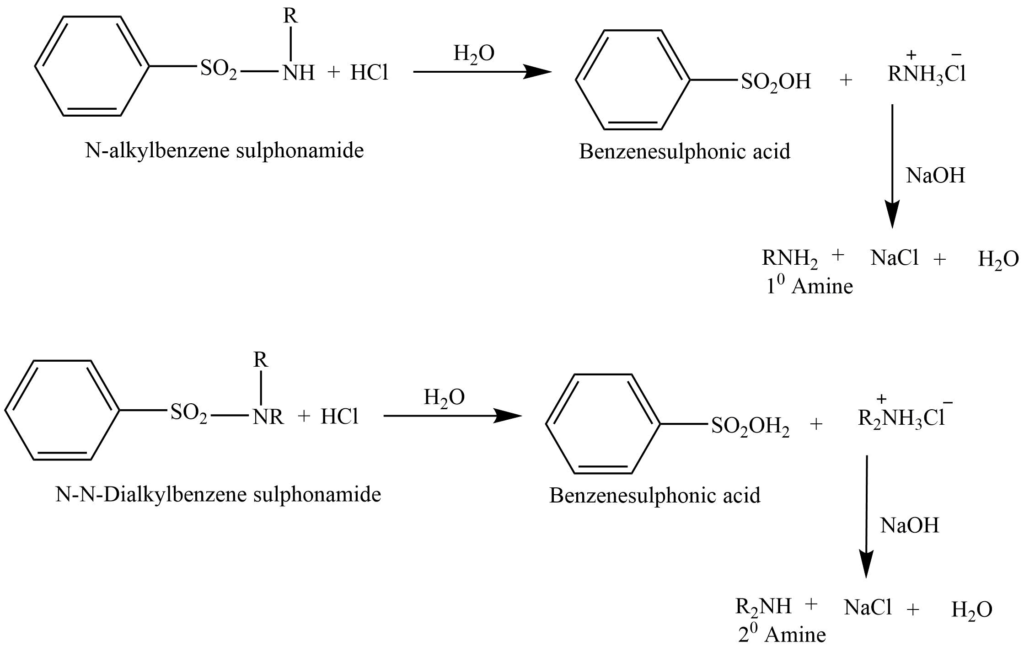
Hinsberg method
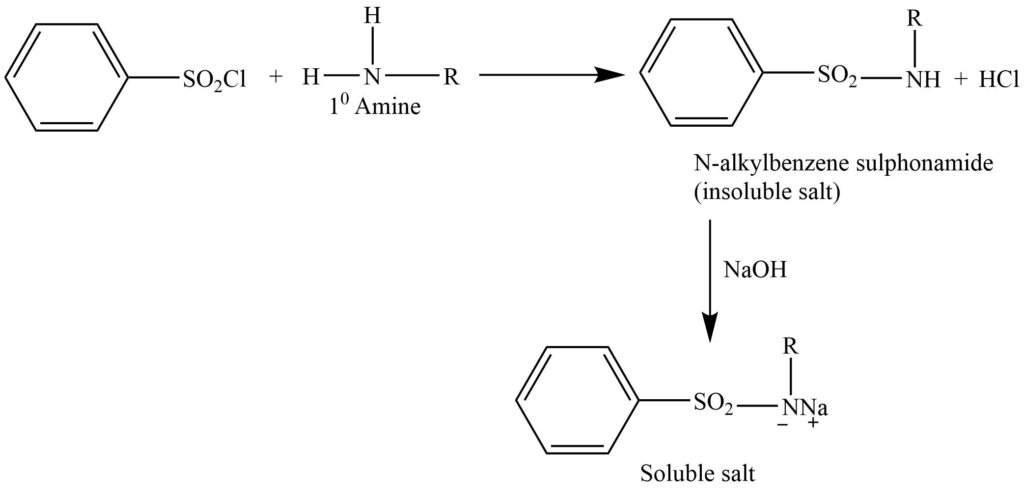
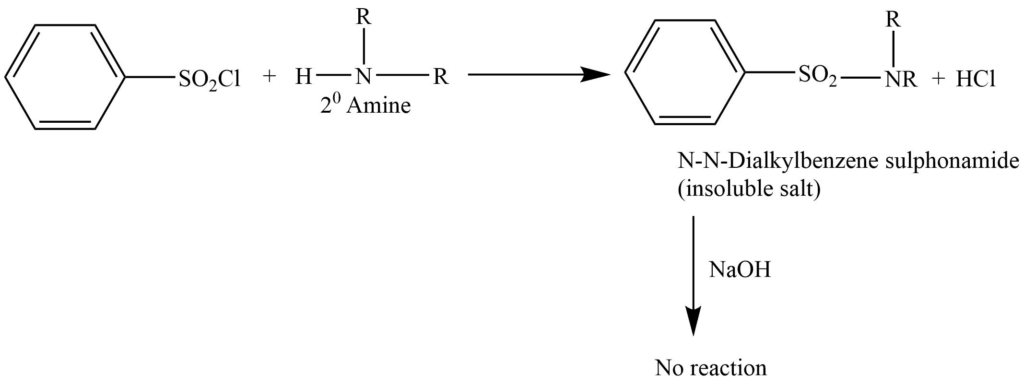
This method involve the reaction of benzenesulphonyl chloride (Hinsbergnberg reagent) , then the solution is made alkaline with an aqueous NaOH solution.
a. Primary amine gives N-alkylbenzenesulphonamide, which forms a salt with NaOH, that is soluble in water.
b. Secondary amine gives N,N-dialkylbenzenesulphonamide. This doesn’t react with NaOH and is insoluble in an alkaline solution.
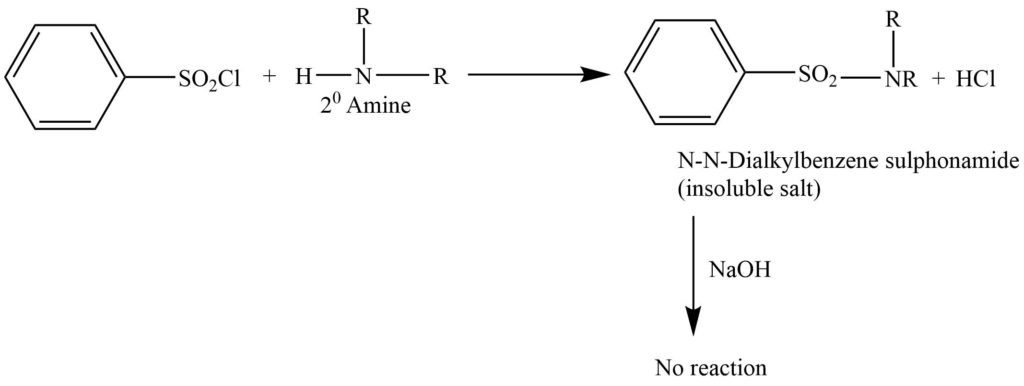
c. The tertiary amine doesn’t react.
Tertiary amine passes over on the distillation of the remaining alkaline solution and the remaining mixture is filtered. The filtrate on further acidification gives sulphonamide of primary amine while sulphonamide of secondary amine remains as solid. These two sulphonamides hydrolyzed with conc. HCl and distilled over NaOH to yield the respective amines.
Physical properties of Amines
- Lower amino acids are gaseous in nature and possess a characteristic ammonia-like smell.
- Primary amines with three or four carbon atoms are liquid at room temperature while the higher ones are solid.
- Normally amines are colorless. They get easily oxidized and develop color due to impurities.
- Amines have higher boiling points compared to non-polar compounds having the same molecular weight.
- Lower aliphatic amines are soluble in water, as they are capable of forming hydrogen bonds with water. The solubility decreases with an increase in the size of the alkyl hydrophobic alkyl group increases.
- In the IR spectrum amine shows characteristic N-H stretching at 3330-3570 cm-1. In this region, the primary amine show two sharp peaks, the secondary amine show only one, and the tertiary amino doesn’t show N-H bonds. The N-H bending frequency occurs at 1550-1650.
Chemical properties of Amines
The main reactions of amines are due to the presence of lone pair of electrons on the nitrogen that is available for donation to the electron-seeking reagent. Amines are nucleophilic reagents.
Salt formation
Amines are basic compounds, that react with mineral acids to form salt.
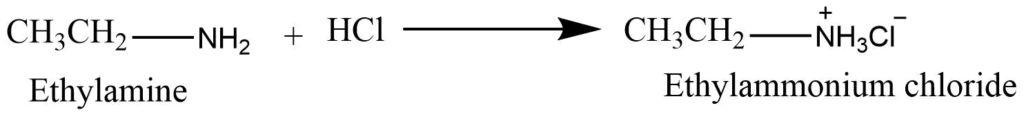
These salts, on reaction with NaOH produce the parent amines.

Reaction with alkyl halide
Alkyl halide reacts with amine, where the hydrogen atoms bonded to the nitrogen atom are successively replaced by the alkyl group. At last, the tertiary amine adds one molecule of the alkyl halide to form quaternary ammonium salt.
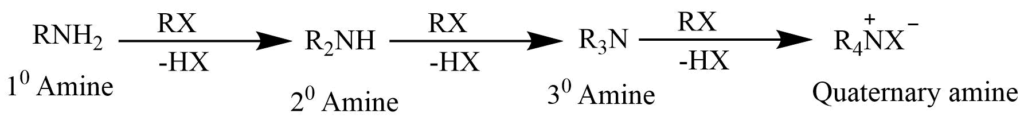
Reaction with an acid chloride (acylation)
a. Primary amines react with the acid chloride or acid anhydride to form N-substituted amides.
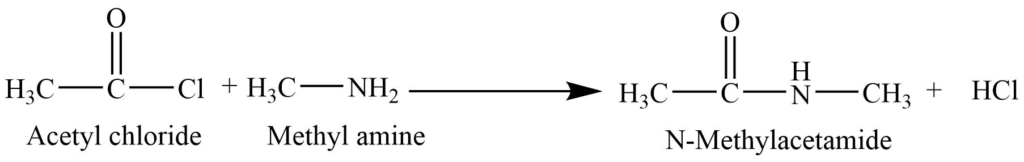
b. Secondary amines react with acid chloride to form N-N’- disubstituted amides.
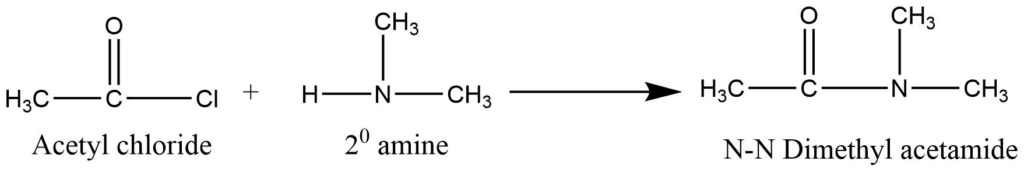
c. Tertiary amines do not show the acylation reaction as they do not have replaceable hydrogen on the nitrogen.
Reaction with nitrous acid
a. Primary amines react with the nitrous acid to form alcohol and nitrogen gas.
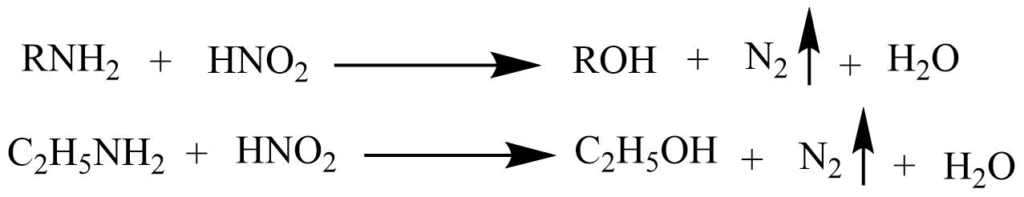
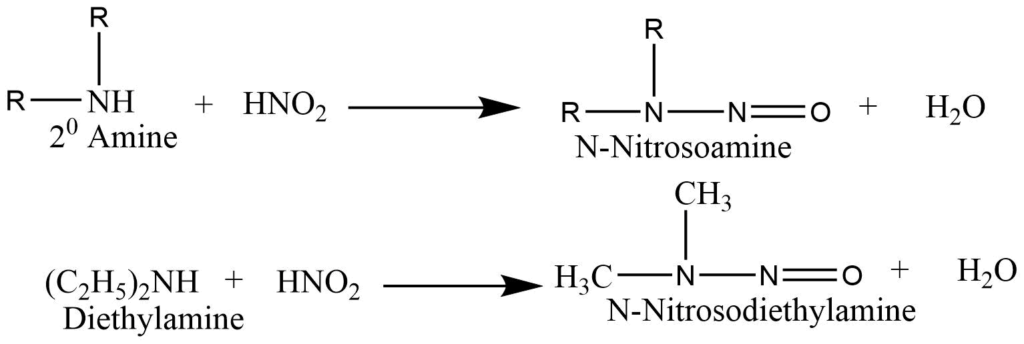
b. Secondary amine reacts with nitrous acid to form N-nitrosamines that form a yellow oily layer, soluble in water.
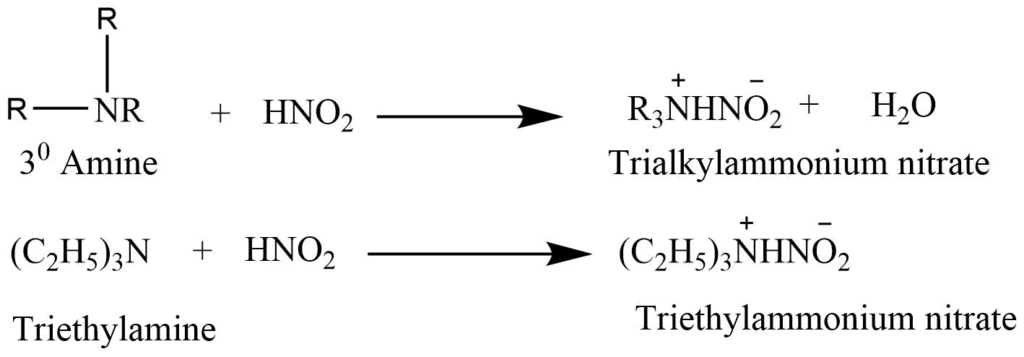
c. Tertiary amines react with nitrous acid to form trialkylammonium nitrite salts that are soluble in water.
Rection with the benzene sulphonyl chloride (Sulphonylation )
a. Primary amine reacts with benzene sulphonyl chloride to form N-alkylbenzenesulphonamide. The sulphonamides from the primary amines have replaceable hydrogen on the nitrogen. This hydrogen is acidic. Thus this sulphonamide dissolves in bases to form a soluble salt.
b. Secondary amines react with benzene sulphonyl chloride to form N-N dialkylbenzene sulphonamide. C. Tertiary amines don’t react with benzene sulphonyl chloride as they don’t possess replaceable hydrogen
Uses of Amines
- Quaternary amines are used in conditioners, detergents, and antimicrobial agents.
- In gas treatments, amines are used to remove CO2 from combustion gas.
- They act as important ingredients in the preparation of material dyes.
- They use as a developing agent in photographs.
- They are used to solubilize herbicides.
- They are used to make personal care products, different drugs, and also in water purification.
References
- Arun Bahl, and B.S. Bahl (2006). A textbook of organic chemistry Chemistry. New delhi: S. CHAND.
- https://byjus.com/chemistry/amines/
- https://byjus.com/jee/amine/
- https://byjus.com/chemistry/physical-properties-of-amines/
- https://www.britannica.com/science/urea
- https://www.chemguide.co.uk/organicprops/amines/background.html
- https://www.vedantu.com/chemistry/physical-properties-of-amines
- https://www.geeksforgeeks.org/physical-properties-of-amines/
- https://www.vedantu.com/chemistry/uses-of-amines
