Green chemistry is the practice of chemical science and manufacturing in a way that is sustainable, safe, and non-polluting, consuming the least amount of materials and energy while producing little or no waste. Applications of Green Chemistry include the applicability of green techniques in the industry, drug development, agriculture, and so on Green chemistry begins with the recognition that the incorrect production, processing, use, and eventual disposal of chemical products can cause harm. To achieve its goals, green chemistry, and green chemical engineering may modify or completely redesign chemical products and processes to reduce waste and the use or generation of particularly hazardous materials. Green chemistry, far from being economically regressive and a drag on profits, is about increasing profits and encouraging innovation while protecting human health and the environment.

Green chemistry is commonly used in the chemical, pharmaceutical, paper, polymer, clothing, and color industries. It is also important in various energy sciences and the development of novel techniques for producing solar cells, fuel cells, and batteries for energy storage. Green chemistry is also widely used in nanoscience and technology. Because the primary goal of green chemistry is to reduce or eliminate waste in the chemical industry, it has inspired the development of numerous green “next generation” catalysts.
Interesting Science Videos
Applications of Green Chemistry
Green Chemistry in Pharmaceutical Industry
Green chemistry is important in the pharmaceutical industry and is causing a revolution. Pharmaceutical companies are making significant improvements in the production of drug molecules while also saving the environment through the use of green chemistry. These methods are more effective and less toxic, and they have the potential to benefit millions of patients.
BASF, a chemical company, now produces ibuprofen (painkiller) in three steps rather than six. Zocor (simvastatin), a leading drug for the treatment of high cholesterol, is traditionally synthesized using a multistep method involving large quantities of hazardous reagents, resulting in a large amount of toxic waste. Codexis, a bio-catalysis company, created a new method for synthesizing the drug using an engineered enzyme and a low-cost feedstock. Paclitaxel (marketed as Taxol) was extracted from yew tree bark, a process that required a large amount of solvent to kill the tree. The drug is now produced in a fermentation vat by growing tree cells.
Examples of Drug Synthesized Using Green Chemistry
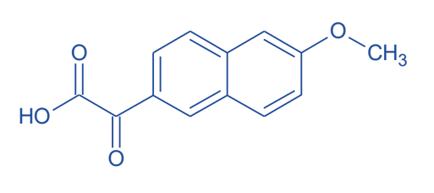
- Naproxen is synthesized with a chiral metal catalyst containing BINAP (2,2’- bis(diphenylphosphino)-1,1’binaphthyle) ligand with good yield and mild reaction procedure.
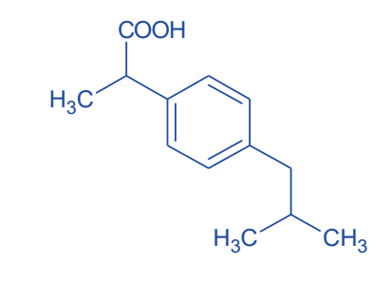
- Ibuprofen is synthesized in a novel manner, which reduces the production of undesirable byproducts. This new method of producing ibuprofen (a nonsteroidal anti-inflammatory drug) is based on “Green Chemistry” principles. In 1997, this improved synthesis received the President Green Chemistry Challenge Greener Synthetic Pathways Award.
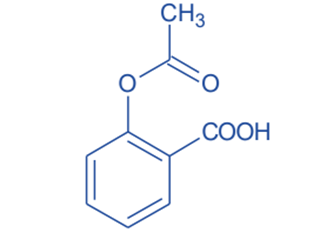
- Aspirin was synthesized using a solvent-free approach. This study demonstrates a new, greener alternative to the conventional synthetic procedure.
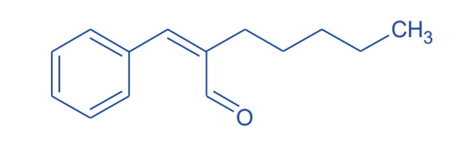
- To prevent the formation of acids from aldehyde, jasminaldehyde is synthesized through a condensation reaction between 1-heptanal and benzaldehyde in a 1:5 ratio in the presence of chitosan as a catalyst in a nitrogen atmosphere. This method has a shorter reaction time and produces no toxic waste.
Energy science
Organic solar cells are a low-cost and potentially environmentally friendly source of energy (OSCs). OSCs are developed using π- conjugated molecules and polymers. Green chemistry principles are applied to the synthesis of conjugated systems. From this standpoint, scientists prefer methods with fewer steps and the use of bio feedstock.
Eco-Friendly Dry Cleaning of Clothes
Per chloro ethylene (PERC) is a cancer-causing agent that is used in dry cleaning. To address this issue, Joseph De Simons, Timothy Romark, and James invented supercritical CO2 and a surfactant for cleaning garments, and Micell Technology created a metal cleaning framework that uses CO2 and a surfactant to replace halogenated solvents.
Green Chemistry in Research Laboratories
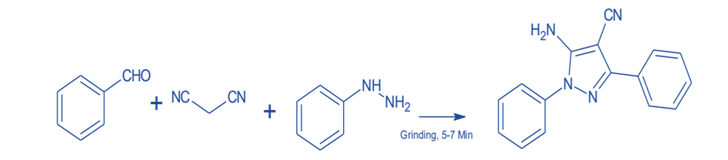
Using green chemistry principles, researchers and chemists have used a large number of biocatalysts and green catalysts in the synthesis of chemical compounds. Green synthetic methods provide clean and safe pathways for reaction mechanisms. The simple grinding, catalyst-free, one-pot, three-component system was used to synthesize polysubstituted amino pyrazoles. This method revealed that the majority of pyrazole synthetic strategies involve multi-step sequences, costly catalysts, anhydrous conditions, an inert atmosphere, and long reaction times.
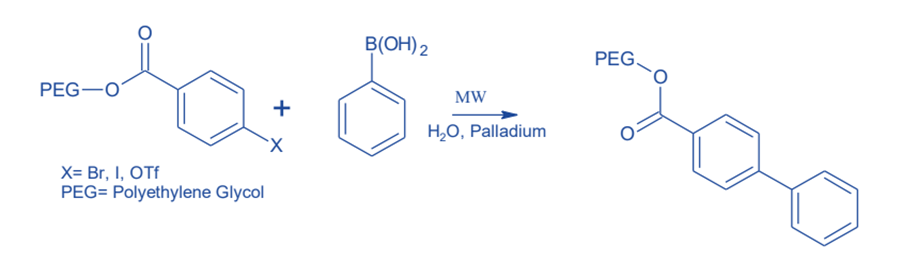
On solid-phase PEG, highly fluorinated compounds are synthesized. This is a safe microwave-assisted reaction, as well as PEG is a soluble polymeric support for small molecule synthesis that allows for easy purification after the reaction.
Clearing Turbid Water
Alum intensify poisonous ions in treated water and cause diseases such as Alzheimer’s. Tamarind seed kernel powder, discarded as agricultural waste, is used to clear and purify municipal and industrial wastewater. Kernel powder is non-poisonous, non-perishable, and less expensive than Al-salt for treating such water.
Catalyst design
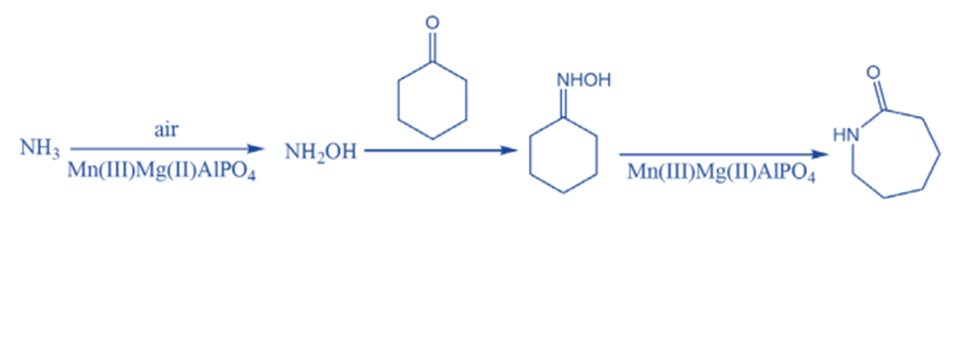
There are numerous opportunities to improve traditional industrial processes. A new route to the Nylon-6,6 precursor o-caprolactam, for example, has been developed using nanoporous aluminophosphate catalysts with a distribution of acidic and redox active sites. Thus, one-step, solvent-free reaction eliminates hazardous reagents and reduces waste byproduct generation.
Nanoscience applications
Nanomaterials have a wide range of applications across all disciplines. Researchers are working to create low-dimensional materials that can be used in a variety of technical applications. Nanotechnology is also concerned with the environment. Mechanical and chemical methods for wastewater treatment as well as air purification using nanofiltration techniques are being developed. In recent years, one of the important methods for the synthesis of low-dimensional materials has been the green chemical approach.
Certainly, green chemical processes deserve credit for reducing agent selection, avoiding surfactants, and improving yields, size distribution, and purity. In the context of green chemistry, there are chemical synthetic approaches such as the citrate method, the tollens method, the ionic liquid method, the polysaccharide method, the ligand exchanging method, and the polyoxometalate method. A few decades ago, the reduction of gold salts by citrate anions was established, yielding nearly monodispersed gold particles in the nano range. Sucrose ester micellar-mediated synthesis of Ag nanoparticles, with NaOH, was added which then speed up the formation of Ag nanoparticles. Carboxymethylcellulose (CMC) derivatives were used to play a dual role in the formation of silver nanoparticles, acting as a reducing agent for silver ions as well as a stabilizing agent.
Green Chemistry in Our Daily Lives
Green chemistry also plays an important role in our daily lives, as demonstrated by the brief examples provided below. Perchloroethane (PERC) is a solvent that is commonly used in dry cleaning. Joseph and colleagues created a new technology known as Micell technology. For dry cleaning, they used liquid CO2 accordingly as a solvent and surfactant in this technology. This new dry-cleaning concept thus eliminates the use of halogenated solvents.
Researchers are currently using used and exhausted vegetable oil as a fuel for vehicles by making very few modifications to the cars currently in use, and hence a startling result was discovered: by using vegetable oil, CO2 emissions were reduced by nearly 67% without affecting vehicle efficiency. Also, wood-dissolving solvents in paper manufacturing, sodium hydroxide (NaOH) and sodium sulfide (Na2S) are used.
This process decomposes approximately 80-90% of lignin. For good-quality paper, the remaining lignin is removed by chlorine gas (Cl2), but this Cl2 also reacts with the aromatic ring of lignin and forms dioxins, which are highly carcinogenic. These halogenated compounds enter the food chain and thus are harmful to living things. As a result, green chemistry offers a variety of environmentally friendly bleaching agents such as hydrogen peroxide (H2O2), ozone (O3), and oxygen (O2). These bleaching agents are hence used in laundry, resulting in less water consumption
In Environmental Safety
The use of plastic contributes to increased pollution in the environment. Plastic particles and other pollutants derived from plastic have been discovered in our environment and food chain, posing a health risk. Hence, biodegradable plastics material aims to create a more sustainable and greener world with a lower environmental footprint.
This evaluation should consider the goals and priorities for producing a diverse range of biodegradable polymers over their entire life cycle. As long as proper waste management, such as composting, is implemented, biodegradable plastics can have features similar to standard plastics while also providing additional benefits due to their lower carbon dioxide impact on the environment.
Lactic acid production optimization
Lactic acid is the starting material for poly-lactic acid. The study’s goal was to create lactic acid from agro-industrial waste as a low-cost, renewable substrate while also reducing pollution levels in the environment. Additionally, sixteen bacterial isolates were isolated from agro-industrial waste. Agricultural as well as industrial wastes were chemically hydrolyzed using hydrochloric acid, sulfuric acid, and sodium hydroxide.
The highest lactic acid yield was discovered using 16S rRNA. As a result, the optimal conditions for the production of lactic acid were discovered. Calcium sulfate was precipitated from calcium lactate produced during culture fermentation using sulfuric acid. The filtrate containing free organic acid was evaporated to produce pure lactic acid.
References
- https://www.organic-chemistry.org/topics/green-chemistry.shtm
- Green chemistry applications: A brief review on variety of uses P-ISSN: 2349–8528, E-ISSN: 2321–4902, IJCS 2018; 6(1): 1517-1522
- https://www.embibe.com/exams/green-chemistry/
- Ahluwalia VK, Kidwai M. New Trends in Green Chemistry, Anamaya publisher, New Delhi, 2004.
- Zhang W, Cue BW. A text book on Green Techniques for Organic Synthesis and Medicinal Chemistry, 2012.
- https://www.epa.gov/greenchemistry/basics-green chemistry#:~:text=Green%20chemistry%20aims%20to%20design,reducing%20pollution%20at%20its%20source.&text=*Chemicals%20that%20are%20less%20hazardous,Less%20toxic%20to%20organisms
- Anastas PT, Warner JC. Green Chem Theory and Practice. Oxford Univ. Press, New York, 1998.
- Anastas PT, Hovarsth IT. Innovations and Green chemistry, Chem. Rev. 2007; 107:2169.
- Ravichandran S. Int. J. Chem Tech Res. 2010; 2(4):219 Trost BM. Atom economy-A challenge for organic synthesis: Homogenous catalysis leads the way. Angew
Chem Int., Ed. 1995, 34:259, - Sheldon RA. Green Solvents for sustainable organic synthesis: State of the art. Green Chem. 2005; 7:267 Bharti VB. Resonance. 2008, 1041.
