Interesting Science Videos
What is Aspartic Acid (Aspartate)?
Definition- Aspartic acid or aspartate, also known as amino succinic acid is a non-essential amino acid that is synthesized itself in the human body through different sources of foods. It is mainly responsible for synthesizing proteins and regulating hormones so also known as building blocks.
Aspartic acid is involved in synthesizing four different amino acids as it plays a vital role in Kreb’s cycle; methionine, isoleucine, lysine, and threonine. It is an aspartate family and a proteinogenic amino acid. It is also a neurotransmitter.
Aspartic acid is the product formed by the hydrolysis of proteins. Aspartic acid was first identified in 1868 from legumin in plant seeds. As aspartic acid are non-essential amino they are synthesized in the body from oxalo acetic acid that is produced during the metabolism of carbohydrates.
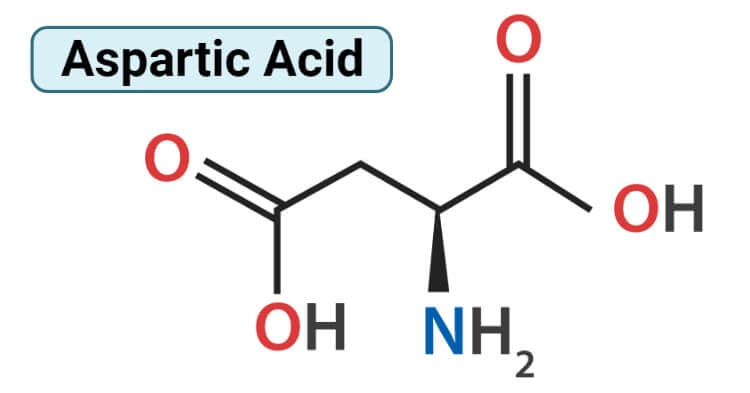
Structure of Aspartic Acid (Aspartate)
Aspartic acid consists of two functional groups, one amino group is basic in nature and the other is the acidic carboxyl group. Therefore, amino acids molecule exists as a zwitterion. Aspartic acid is alanine with one of the β hydrogens replaced by a carboxylic acid group. The pKa of the β carboxyl group of aspartic acid in a polypeptide is about 4.0. It is a dibasic amino acid having two carboxyl groups; one on alpha carbon atom and another on the side chain. Aspartic acid has an alpha-keto homolog. Aspartic acid is divided into two forms; L-aspartic acid and D-aspartic acid. L configuration is a more common and dominant form. L-aspartic acid is typically involved in the production of antibodies and is part of protein synthesis in the body which is responsible for increasing the immune system. D-aspartic acid is not involved in protein synthesis and is mainly found in the pituitary gland and testes which is used in the regulation, synthesis, and release of testosterone and luteinizing hormone.
Sources of Aspartic Acid (Aspartate)
- It is found in sugar cane and sugar beets molasses, asparagus, avocado, sprout seeds, and oat flakes.
- Animal Sources includes: Sausage meat, Luncheon meat and wild game.
- Other sources includes magnesium aspartate that is salt of aspartic acid and sweeter aspartame.
Physical Properties of Aspartic Acid (Aspartate)
- Molecular weight: 133.10
- White, crystalline solids
- Polar
- Acidic
- Hydrophilic
- Orthorhombic, bisphenoidal leaflets or rods
- Sour in taste
Chemical Properties of Aspartic Acid (Aspartate)
- Melting point: 270ºC
- Solubility: 5390 mg/L at 25 ºC
- Density: 1.6603 at 13 ºC
- LogP: -3.89
- pKa: 2.77 because of two carboxyl molecule
Biosynthesis of Aspartic Acid (Aspartate)
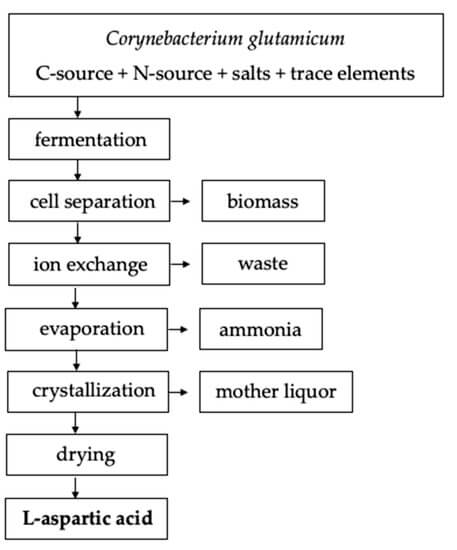
Protein extraction, chemical synthesis, and enzymatic conversion are three main methods to produce aspartic acid. A large number of amino acids are produced in the extraction method from the hydrolysis of protein. In this method, L-aspartic acid should be separated. Chemical synthesis requires high temperature and pressure in a racemic mixture producing both isomers of aspartic acid. So, enzymatic conversion is the best method for the production of aspartic acid. Bacterial fermentation is the best for the highest yield of amino acids. Pseudomonas, Bacillus, and Proteus are considered as the main producers but E. coli and Corynebacterium glutamacium are mostly preferred by industries.
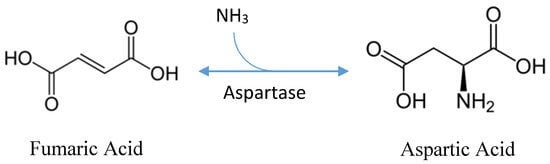
In the 1960s, the fermentation process is developed and patented that utilizes sugar-free medium and uses fumaric acid as a sole source of carbon. Ammonia serves as a nitrogen source that is used in catalysis. Ammonia and fumaric acid are used in combination as 1:1 or 1:2 ratio. the pH of the broth is initialized to 7 and that naturally increases from 8.4 to 9.6 in the initial stage that allows for the production of acid. Fermentation can be done with or without agitation for 2 to 10 days at 27-40 ºC. L-aspartic acid will be secreted and accumulate in the culture broth. Different downstream processes are available to separate L-aspartic acid from the culture broth. But in the case of batch fermentation, ion exchange resins can be used to separate and purify L-aspartic acid followed by crystallization. L-aspartic acid can be separated by adjusting the broth to 90 ◦C and a pH of 2.8 with sulfuric acid in continuous fermentation. After the pH is adjusted to 2.8, the isoelectric point will cause L-aspartic acid to precipitate out of the solution. It is then subjected to a two-hour incubation period at 15 ºC to induce protein crystallization. Under these conditions, L-aspartic acids yield 95%.
Functions and Uses of Aspartic Acid (Aspartate)
- It is easily available multivitamins that are found in different forms as tablets, powders, and fluids.
- Because of its role in regulating testosterone levels, D-aspartic acid is used as increasing muscle mass.
- It also helps in keeping the concentrations of NADH (Nicotinamide adenine dinucleotide) high in brain cells and also increase the mind sharpness leading to further production of neurotransmitters as well as chemicals needed for normal mental functioning.
- Can also be used for increasing fertility.
- It is also used to produce poly aspartic acid that is used as a fertilizer synergist.
- It also aids in energy production, RNA and DNA synthesis, and liver detoxification.
- It also helps in removing excessive toxins from the cells like ammonia.
- It is also used as building blocks molecules for active pharmaceutical agents.
- It is useful in making culture medias, detergents, fungisides and germicides.
Safety and Toxicity of Aspartic Acid (Aspartate)
- Excessive intake leads to a negative nitrogen balance in the body. This can lead to the following effects on the body.
- Anemia
- Lower infection resistance
- Impaired metabolism
- Development of the fatty liver
References
- Appleton H and Rosentratar AK (2021). Sweet Dreams (Are Made of This): A Review and Perspectives of Aspartic Acid Production. Fermentation.7: 49
- From, https://www.news-medical.net/health/What-is-Aspartic-Acid.aspx
- From, https://pubchem.ncbi.nlm.nih.gov/compound/Aspartic-acid#section=Dissociation-Constants
- From, https://www.britannica.com/science/aspartic-acid
- From, https://byjus.com/chemistry/aspartic-acid/
