
Atomic emission spectroscopy (AES) is an analytical technique used to quantify metal atoms by measuring the intensity of light produced by the atoms in excited states. When an excited atom returns to its ground state, it emits a specific wavelength of radiation. Excitation (absorption of radiation) and de-excitation (emission of radiation) of electrons are both involved in atomic emission spectroscopy.
Atomic emission spectroscopy analyzes the radiation released by atoms to identify their structure, composition, and surroundings. We can derive the energy levels (or stationary states) of the atom from wavelength measurements, and this gives an experimental basis for theories of atomic structure.
Interesting Science Videos
Basic principle of atomic emission spectroscopy
AES is based on the idea that when energy is delivered to a molecule in the form of light or heat, molecules are excited and shift from a lower energy level state to a higher energy level state. The molecules are unstable at higher energy levels and revert to lower energy levels after producing radiations in the form of photons. The emission spectrometer measures the wavelengths of emitted photons.
The basic principle of atomic emission spectroscopy is the study of the wavelengths of photons released by atoms and molecules as they move from a high energy state to a low energy state. Each element or substance emits a distinct set of wavelengths that are determined by its electrical structure. The elemental structure of the sample can be revealed by studying these wavelengths.
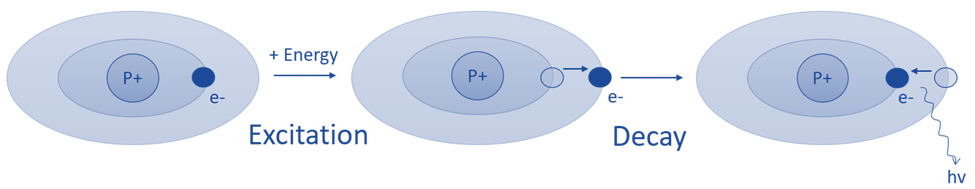
Fig: Basic principle of atomic emission spectroscopy
Instrumentation of atomic emission spectroscopy
Nebulizer
Before analyzing a sample, it should be transformed into highly excited free atoms. The most convenient way to introduce liquids into the gas stream is via aerosol from a nebulizer. The aerosol could be created by the action of a high-speed jet across the tip of the small aperture, or by some other method. The spectral emission’s stability is greatly reliant on droplet sizes. As a result, choosing the right nebulizer type is crucial for producing homogeneous droplet sizes. The appropriate nebulizer depends on sample properties such as density, viscosity, organic content, total dissolved solids, and total sample volume.
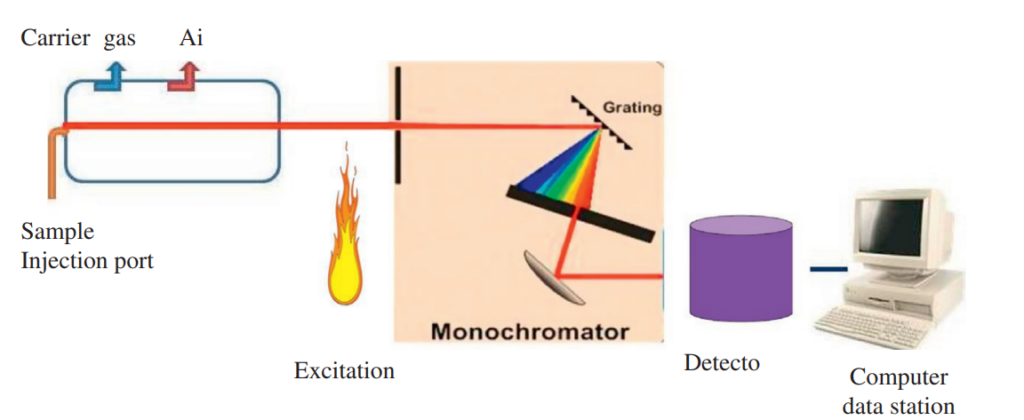
Fig: Instrumentation of atomic emission spectroscopy
Image source: https://www.sciencedirect.com/science/article/abs/pii/B978012814217200010X
Excitation Sources
The sample’s atoms are dissolved, atomized, and excited using an excitation source. The ideal excitation source will allow the excitation of all the elements in the sample and will do so again until the complete elemental excitation in the sample is covered.
Direct-Current Plasma
This excitation method uses two electrodes to generate an electrical discharge that heats the plasma gas, which is commonly argon. This form of stimulation is more suited for materials with a high solids content.
Inductively coupled plasma
This is the most often used excitation method, and it necessitates the use of a plasma torch made from concentric quartz tubes to create excitement in the material. The light source for inductively coupled plasma atomic emission spectroscopy (ICP-AES) is a high-frequency inductively coupled plasma.AES uses wavelengths between the upper vacuum ultraviolet (160 nm) to visible light (800 nm). It entails the excitation and de-excitation of electrons by radiation absorption.
• Electromagnetic radiation (EMR) is measured and evaluated when an electron transitions from an excited to a de-excited state.
• The optical quality of radiation during the de-excitation process gives rise to the name optical emission spectroscopy (OES). Because the spectral line has a fixed wavelength, measurement in atomic emission spectroscopy is possible.
Flame
In this procedure, a sprayed solution or gas containing a sample of the substance to be examined is exposed to flame. When the flame heat evaporates the solvent and destroys the chemical bonds of the analyte, free atoms of the substance are created. Heat also causes atoms to become electrically charged particles, which emit light when they return to their ground electronic state. The flame photometer principle is based on measuring the intensity of emitted light when a metal is introduced into the flame. The wavelength of the color indicates the element, and the color of the flame indicates the number of elements present in the sample.
Microwave Induced Plasma (MIP)
Frequencies in the microwave region are employed as an external energy source in a microwave induced plasma (MIP) source. It is typical to use microwave radiation with a frequency of 2450 MHz. When ultrahigh frequency ac power is capacitively coupled into a stream of noble gas (helium or argon) or nitrogen at around 3 dm3 /min in a resonant cavity, a microwave induced plasma is formed.
Laser-Induced Plasma (LIP)
The heated plasma is maintained in this process by a support gas, commonly argon, which is focussed by a high-energy CO2 laser source.
Arc or Spark
Spark and arc excitation sources use a spark, an electric pulse, or a continuous electrical discharge arc between two electrodes to vaporize and excite the sample’s atoms.
Monochromator
Monochromators are prisms and diffraction gratings. These are used to select the specific kind of radiation generated by the analyte and to remove any other undesirable radiation. As a result, it is also known as ‘the wavelength selector’. Diffraction gratings produce more accuracy and resolution than prisms.
Gratings are utilized as a dispersive element in the AES spectrometer to disperse incident light into component wavelengths. The light is reflected off the angled grating surface, causing wavelengths to be distributed through constructive interference at wavelength-dependent diffraction angles. Because all atoms in a sample are excited at the same time, they can be detected sequentially using a monochromator or simultaneously using a polychromator with multiple detectors.
Detectors
Detectors are transducers that convert the spectrometer’s analog output into an electric signal that can be seen and processed on a computer. In atomic emission spectroscopy, detectors include photomultiplier tubes (PMTs), charge-coupled devices (CCDs), and charge-injection devices (CIDs). They convert optical impulses into electrical current, which the amplifier subsequently amplifies.
Amplifier
The amplifiers receive signals from the detectors and amplify them multiple times such that they are usable or comparable.
Readout device
Atomic emission spectroscopy employs computers as readout devices. Using the atomic emission ranges library, computers analyze the data in the form of spectra and plot the calibration curve.
Advantages of atomic emission spectroscopy over Atomic absorption spectroscopy
1. Lower interelement interference due to increased temperature.
2. Emission spectra are created by using a single set of excitation conditions, and several elements can be recorded at the same time.
3. Multielement analysis can be performed on relatively small samples.
4. Refractory substances with low concentrations can be identified.
Applications of atomic emission spectroscopy
i. The ICP-AES technology can be used in agriculture to analyze agricultural and food goods.
ii. It can be used in earth science to analyze rare earth elements found in rocks.
iii. The ICP-AES technique can examine trace metals from alloys, steel, lubricating fluids, and gasoline.
iv. Similarly, in biology, the ICP-AES technology can evaluate aluminum from blood, copper from brain tissue, selenium from the liver, and salt from breast milk.
v. Inductively coupled plasma atomic emission spectroscopy can detect metal traces such as calcium (Ca), copper (Cu), iron (Fe), manganese (Mn), magnesium (Mg), phosphorus (P), potassium (K), and zinc (Zn) in beer or wine.
vi. The quantities of Na+ and K+ ions in the human body are critical for performing numerous metabolic tasks. The quantities of these substances can be measured by diluting and aspirating a blood serum sample into the flame.
vii. Flame photometry can be used to determine the amounts of various metals and elements in soft drinks, fruit juices, and alcoholic beverages.
viii. The amounts of various metals and elements in soft drinks, fruit juices, and alcoholic beverages can also be determined using flame photometry.
ix. AES is used to determine calcium and magnesium in cement.
x. AES is used to determine lead in gasoline.
xi. Ca, Mg, Na, and K levels in blood serum and plasma were analyzed by using AES.
xii. AES is an effective method for detecting metallic toxins.
xiii. AES can determine the metallic contents of the soil.
xiv. AES can be used to detect adulteration in petroleum products.
Advantages of atomic emission spectroscopy
1. It is a sensitive method capable of detecting concentrations as low as 1 ppm.
2. The analysis requires a small sample.
3. If proper comparison standards are provided, working time is reduced.
4. There is no need to prepare the sample.
5. Solid and liquid samples are easily examined.
Disadvantages of atomic emission spectroscopy
1. It only applies to metals and metalloids. Nonmetals cannot be examined.
2. The instrument is quite expensive.
3. It is a destructive procedure that results in the destruction of the sample.
4. Concentrated solutions are undetectable.
References
- Thirumdas, R., Janve, M., Siliveru, K., & Kothakota, A. (2019). Determination of food quality using atomic emission spectroscopy. In Evaluation Technologies for Food Quality (pp. 175-192). Woodhead Publishing.
- https://www.sciencedirect.com/science/article/abs/pii/B9780444517340500053.
- https://forensicyard.com/atomic-emission-spectroscopy-aes/
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_2.1_(Harvey)/10%3A_Spectroscopic_Methods/10.07%3A_Atomic_Emission_Spectroscopy.
- https://www.chemistrylearner.com/atomic-emission-spectroscopy.html.
- https://soe.unipune.ac.in/studymaterial/ashwiniWadegaonkarOnline/CH%20%208%20and%2010%20-Basic%20Principles%20of%20Atomic%20Absorption%20and%20Atomic%20Emission%20Spectroscopy.pdf
