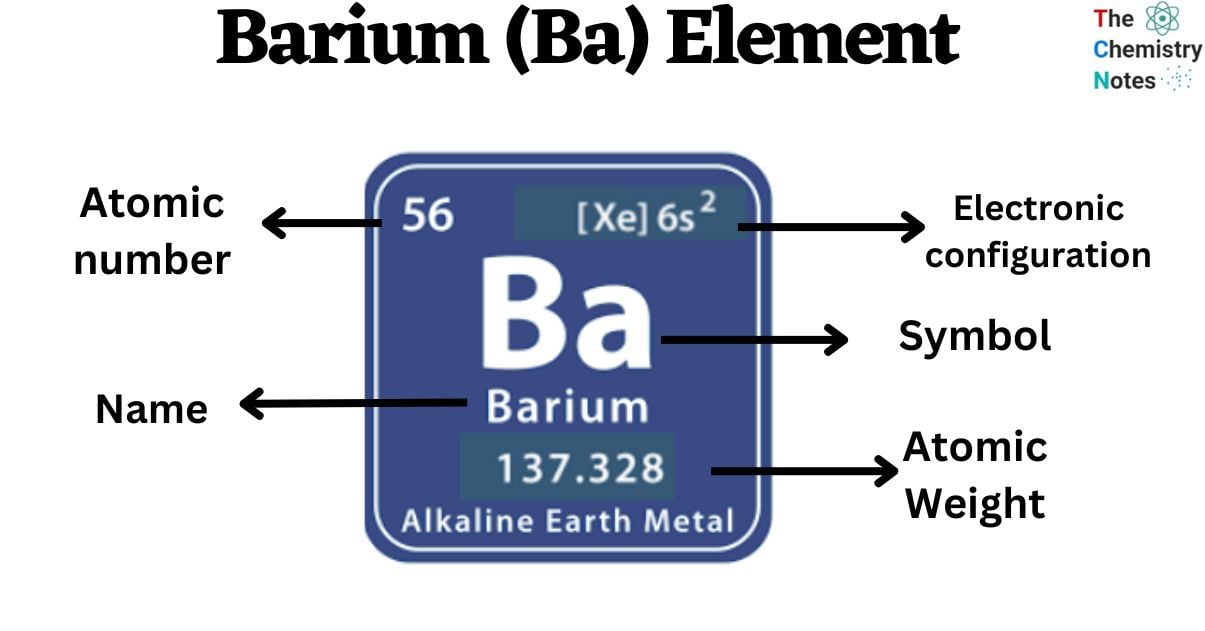
Barium is a chemical element with the atomic number 56 and is represented by the symbol ‘Ba’ in the periodic table. It is soft and silvery in appearance and classified as alkaline earth metal and belongs to the s-block of group 2 of the periodic table. It is a highly reactive chemical so it can not be found as a free element in nature.
Barium makes up 0.425 percent of the Earth’s crust and it is found in different minerals in nature. It is impossible to find barium in its free state since the element always combines with sulfur, carbon, or oxygen. Baryte, also known as barium sulfate (BaSO4), and witherite, also known as barium carbonate (BaCO3), are the two types of barium minerals that are most commonly found.
Interesting Science Videos
History of Barium
- At the beginning of the 1600s, a shoemaker named Vincentius Casciorolus who had an interest in alchemy was quite enthusiastic. He had heard that mountains that were close to Bologna, Italy, had an unusual mineral that was heavy, white with silver flecks, and had exceptional powers. He pondered the possibility that it was in fact the so-called philosopher’s stone.
- Unfortunately for Casciorolus, despite the fact that the material’s qualities were intriguing, it was unable to transform different metals into gold and wasn’t able to grant him immortality. Instead of being referred to as the “philosopher’s stone,” it soon developed a reputation as the “Bologna stone,” which means curiosity.
- Swedish scientist, Carl Wilhelm Scheele, discovered barium for the first time in 1774. However, Scheele worked with barium sulfate (BaSO4), a compound of barium. In fact, barium sulfate is the most common ore of barium found in nature. It is most commonly referred to as barite or barytes.
- In 1808, English chemist Sir Humphrey Davy first isolated barium metal in London. After hearing from Swedish chemist Jacob Berzelius that barium sulfate might be broken down using electrolysis, Davy gave it a try. This was right, and Davy used a similar method to separate strontium.
Occurrence of Barium
- Ba makes up 0.425 percent of the Earth’s crust and is found in different rocks in nature. Barium is never found on its own because it always mixes with sulfur, carbon, or oxygen.
- Baryte, which is also known as barium sulfate (BaSO4), and witherite, which is also known as barium carbonate (BaCO3), are the two types of barium minerals that are most usually encountered. Both of these minerals include barium.
- The Ba2+ ion is present in seawater, with an average concentration of 109 nmol/kg. Additionally, barite, or BaSO4, is another form in which Ba exists in the ocean. Ba exhibits a nutrient-like profile and has a long residence time of 10,000 years.
- Electrolysis of molten barium chloride, BaCl2, is the primary method used in the industrial production of barium.
- China, India, Morocco, Turkey, and Kazakhstan are among the top in the world when it comes to the extraction of barium ores.
- There are 37 distinct isotopes of barium. The barium that occurs in nature is a mixture of seven different isotopes that are stable.
Isotopes of Barium
Barium consists of seven naturally occurring stable isotopes: 130Ba, 132Ba, 134Ba, 135Ba, 136Ba, 137Ba, and 138Ba.
Naturally Occurring Stable Isotopes of Barium
| Isotopes | Natural Abundance (atom %) |
|---|---|
| 130Ba | 0.106 (1) |
| 132Ba | 0.101 (1) |
| 134Ba | 2.417 (18) |
| 135Ba | 6.592 (12) |
| 136Ba | 7.854 (24) |
| 137Ba | 11.232 (24) |
| 138Ba | 71.698 (42) |
Elemental Properties of Iron
| Electronic Configuration | [Xe] 6s2 |
| Atomic Number | 56 |
| Atomic Weight | 133.33 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 2, 6, s-block |
| Density | 3.59 g.cm -3 at 20 °C |
| Ionic radius | 0.135 |
| Van der Waals radius | 0.222 nm |
| Electron shells | 2, 8, 18, 18, 8, 2 |
| Electrons | 56 |
| Protons | 56 |
| Neutrons in most abundant isotope | 82 |
Physical Properties of Barium
- Ba is a soft metal that often has a color appearance between gray and white. A golden hue is imparted to barium which has been thoroughly processed to its highest level.
- Ba has an atomic number of 56. It has a melting point of 727 °C (1341 °F) and a boiling point of 1845 °C (3353 °F).
- It has a solid phase density of 3.51 g/cm3 and a liquid or molten phase density of 3.338 g/cm3.
- It is possible to beat it into thin sheets without it breaking, indicating that it possesses some malleability.
- Ba tarnishes quickly in the air. After being cut, it immediately turns a dark black color due to the creation of barium-oxide, and is denoted by the chemical formula BaO.
- Ba adopts a cubic crystal lattice. This metallic element exhibits a notably low hardness and exhibits a pronounced thermal expansion upon heating.
- Upon heating, barium compounds emit a faint yellowish-green hue. This particular property is employed as a means of conducting a test for the presence of barium.
- It is an excellent conductor of electricity.
- In chemical compounds, barium typically manifests as Ba2+ in its divalent state.
| Color/physical appearance | Lustrous, soft, silvery |
| Melting point/freezing point | 1000 K (727 °C, 1341 °F) |
| Boiling point | 2118 K (1845 °C, 3353 °F) |
| Density | 3.51 g/cm3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 0.89 (Pauling Scale) |
Chemical Properties of Barium
- Ba is highly reactive when exposed to air or oxygen at room temperature.
- It reacts violently with liquids like water or alcohol.
- When treated with chalcogens, barium releases a great deal of energy. This is a strong exothermic process.
- The reactivity of barium metal towards acids is notably high. The compound exhibits solubility in a wide range of aqueous acids. Except for sulfuric acid, where an insoluble protective layer of barium sulfate is formed, thereby accounting for the observed phenomenon.
Chemical Reaction of Barium
- The Reaction of Barium With Air
Upon ignition, Ba metal undergoes combustion in the presence of air, resulting in the formation of a composite of white barium oxide (BaO) and barium nitride (Ba3N2).
3 Ba (s) + N2 (g) → Ba3N2 (s)
When Ba is exposed to air, it forms a thin layer of passivating BaO on the surface as a result of a reaction with oxygen.
2 Ba (s) + O2 (g) → 2 BaO (s)
Barium oxide is typically produced by heating barium carbonate. It would appear that the superoxide BaO2 is also produced during this reaction.
Ba (s) + O2 (g) → BaO2 (s)
- The Reaction of Barium With Water
The chemical element barium exhibits a high degree of reactivity upon contact with water, resulting in the formation of barium hydroxide (Ba(OH)2) and hydrogen gas (H2).
Ba (s) + 2 H2O (g) → Ba(OH)2 (aq) + H2 (g)
- The Reaction of Barium With Acids
The chemical reaction between Ba and hydrochloric acid results in the formation of barium chloride (BaCl2).
Ba (s) + 2 HCl (aq) → BaCl2 (aq) + H2 (g)
Uses of Barium
Barium has been used in different industrial and commercial industries. Some of the uses are discussed here:
Used As Alloy
- Ba is a highly reactive chemical element that is typically rendered stable through its combination with other elements. The element in question has the ability to create alloys with various other elements, thereby enabling its utilization in diverse applications and functions.
- Ba exhibits the ability to form alloys with various metals, such as lead and tin, to produce soldering alloys that enhance creep resistance. Additionally, it can alloy with nickel to generate spark plugs. Furthermore, the combination of barium with calcium, silicon, aluminum, and manganese results in the production of high-quality steel deoxidizers.
- Ba is utilized as an additive during the steel manufacturing process and as an inoculant for cast iron due to its ability to diminish the size of carbon particles, thereby facilitating the formation of microstructures.
- Ba was a fundamental component of the initial high-temperature superconductors, specifically Yttrium Barium Copper Oxide (YBCO). The cooling process was achieved through the utilization of liquid nitrogen, which possesses a transition temperature of 93 K.
Used In Industries
- Ba finds its application in various industries in diverse forms. Barium sulfate is the most commonly utilized form of barium and serves as an essential constituent across numerous industries.
- The petroleum industry employs the use of Barium sulfate (BaSO4), commonly referred to as mineral baryte, as an insoluble additive in oil well drilling fluid.
- Blanc fixe, a precipitate of Barium Sulphate, is commonly utilized in the paint industry for the production of paints and varnishes. The term “Blanc fixe” originates from the French language and translates to “permanent white.”
- Barium sulfate finds application in enhancing the physical characteristics of polymers, such as epoxies. Calcium carbonate finds application as a filler in the manufacturing of plastics and rubbers and as a coating pigment for papers.
Used In Fireworks
- Barium nitrate is frequently used in pyrotechnics to produce visually appealing fireworks displays. Specifically, it is utilized for adding vibrant hues of apple green or yellow to fireworks.
- Barium monochloride finds its application in pyrotechnics, specifically when a vivid green hue is desired. The aforementioned property finds application in the production of green signal flares.
Health Effects of Barium
- Food and water rarely contain enough barium to cause health problems.
Workers in the barium sector have the greatest risk of barium exposure with additional health risks. Barium sulfate and barium carbonate in the air cause most of their health problems. - The health effects of barium are contingent upon the water solubility of its compounds. Ba compounds that are soluble in water pose a significant hazard. The presence of significant quantities of water-soluble barium has the potential to induce paralysis or fatality.
- Water-soluble barium can cause breathing problems, elevated blood pressure, heart rhythm abnormalities, stomach irritation, muscular weakness, nerve reflex changes, brain and liver edema, kidney and heart damage, and more.
Environmental Effects of Barium
- Some barium compounds generated during industrial operations dissolve easily in water and are found in lakes, rivers, and streams. Barium compounds are water-soluble and can spread far. Barium accumulates in fish and other aquatic species after they consume barium compounds.
- Barium’s insoluble salts with carbonate and sulfate prevent it from moving and posing a problem. Permanent barium compounds are generally found in soil or water sediment. Most soils contain low quantities of Ba. At hazardous waste sites, these levels may be higher.
References
- Emsley, John (2011). Nature’s Building Blocks: An A–Z Guide to the Elements (2nd ed.). Oxford: Oxford University Press. ISBN 978-0-19-960563-7.
- Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (2nd ed.). Oxford: Butterworth Heinemann. ISBN 0-7506-3365-4.
- https://www.rsc.org/periodic-table/element/56/barium
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.121. ISBN 1-4398-5511-0.
- https://www.britannica.com/science/barium
- https://byjus.com/chemistry/barium/
