The amount of oxygen needed by bacteria and other microorganisms to break down organic matter in an aerobic environment is known as the biochemical oxygen demand. Although it is commonly employed as a measure of water’s organic quality, it should be noted that this test lacks precise quantitative accuracy. The measurement of organic pollution in water is typically quantified as the amount of oxygen consumed per liter of the sample over a 5-day incubation period at a temperature of 20°C, commonly referred to as BOD5. This parameter serves as a reliable indicator of the extent of organic pollution in water.

The factors that influence dissolved oxygen levels also have an impact on biological oxygen demand. The quantification of biochemical oxygen demand necessitates the acquisition of two distinct measurements. The first measurement involves the immediate quantification of dissolved oxygen (referred to as the initial measurement), while the second measurement entails incubating the sample in a laboratory setting for a duration of 5 days, after which the remaining amount of dissolved oxygen is determined (referred to as the final measurement). This metric quantifies the quantity of oxygen utilized by microorganisms for the purpose of decomposing the organic substances contained within the specimen over the course of the designated period of incubation.
Interesting Science Videos
What is Dissolved Oxygen?
Dissolved oxygen plays a vital role in the preservation of aquatic ecosystems and the aesthetic integrity of streams and lakes.
- Upon observing the water in a lake, it becomes evident that the presence of oxygen is not visually discernible. Water and air are often perceived as contrasting elements. However, it is worth noting that even typical bodies of water, such as lakes or streams, do contain minute quantities of oxygen, which exist in the dissolved form. Despite the relatively low concentration, typically around ten molecules of oxygen per million molecules of water, dissolved oxygen plays a vital role in natural aquatic environments. The presence of an adequate concentration of dissolved oxygen is essential for sustaining the biodiversity and visual appeal of streams and lakes.
- The maintenance of adequate levels of dissolved oxygen is of utmost importance for the preservation of both the aquatic ecosystem and the visual appeal of streams and lakes. The assessment of the impact of organic matter on the concentration of dissolved oxygen (DO) in a stream or lake is crucial for the management of water quality. The quantification of the decomposition process of organic substances in aqueous environments is assessed through the determination of biochemical or chemical oxygen demand. The measurement of oxygen demand pertains to the quantification of oxidizable substances present in a given water sample, which have the potential to decrease dissolved oxygen concentrations.
- Hot summer temperatures and extra fertilizer can reduce dissolved oxygen in a water body, stressing local aquatic life. Biochemical oxygen demand (BOD) is a water analysis used to study how bacteria and other microbes use oxygen to break down organic materials in aerobic (oxygen-rich) conditions.
Direct detection of dissolved oxygen in water using a dissolved oxygen sensor that has been calibrated is the most accurate method. This sensor is able to directly measure the amount of oxygen that is dissolved in water, either in milligrams per liter or as a percentage of oxygen that is dissolved (%DO).
- It is expected that water at lower temperatures will have a larger concentration of dissolved oxygen as well as a higher percent DO, whereas water that is warmer and more polluted will have a lower concentration as well as a lower percent DO.
- In general, the range of dissolved oxygen concentrations that should be present in healthy water is between 6.5-8 mg/L and 80-120%.
Understanding the relationship between organic matter and the amount of dissolved oxygen in a river or lake is crucial for managing water quality. Waste organic matter in water can be decomposed by aerobic bacteria (bacteria that can only survive in the presence of oxygen) and this process is measured by BOD. Living bacterial organisms, which require oxygen to carry out their decomposition, stabilize or render the waste organic matter unobjectionable. The biological oxygen demand (BOD) is a common water quality indicator used in wastewater treatment facilities
What is Biochemical Oxygen Demand (BOD)?
Biochemical Oxygen Demand, also called Biological Oxygen Demand (BOD), is the amount of oxygen that aerobic microbes need to break down the organic matter in a sample of water at a certain temperature and over a certain amount of time.
- A higher biochemical oxygen demand (BOD) signifies an increased need for oxygen, resulting in reduced availability for oxygen-demanding species to sustain themselves, thereby indicating a decline in water quality. Conversely, a lower biological oxygen demand (BOD) implies a reduced depletion of oxygen from water, resulting in a higher degree of water purity. Cold water has a greater capacity to retain oxygen compared to warmer water. Consequently, during the summer months, the initial levels of dissolved oxygen are typically lower.
- Typically, rivers that are free from contamination exhibit biochemical oxygen demand (BOD) levels that are generally below 1 part per million (equivalent to 1 mg/L). In contrast, untreated sewage tends to possess Biochemical Oxygen Demand levels ranging from 200 to 600 ppm.
A bioassay is what the Biochemical Oxygen Demand test is. The word “biological assay” is sometimes abbreviated to “bioassay,” and it refers to a specific kind of experiment that is conducted in vitro. In most cases, the purpose of a bioassay is to determine how the presence of a particular drug affects a living organism. Bioassays can be qualitative or quantitative. Quantitative bioassays often involve estimating the concentration or potency of a chemical by measuring the biological response that the substance induces.
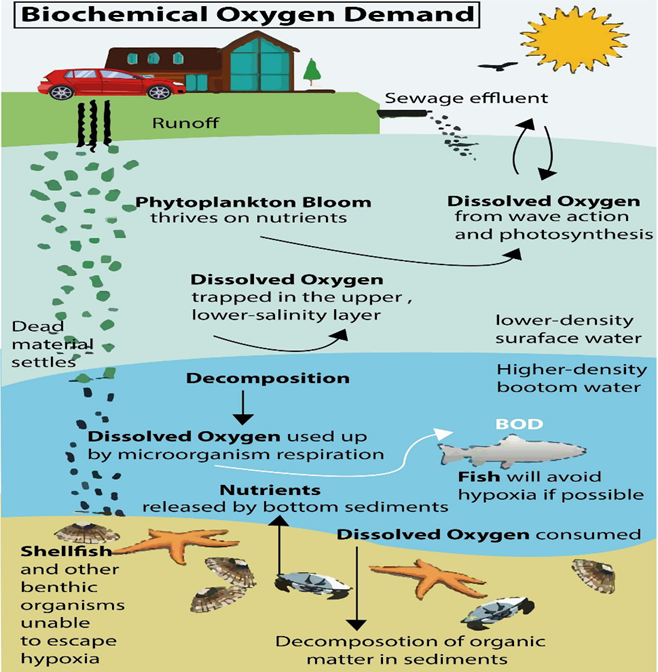
Sources of Biochemical Oxygen Demand
There are various sources that contribute to the increase in biological oxygen. The demand for water encompasses both natural and anthropogenic factors.
- Pollution plays a significant role in the escalation of the biochemical oxygen demand (BOD) in aquatic ecosystems. A healthy lifestyle is characterized by the regular and sufficient consumption of water, which consequently generates a significant volume of wastewater containing organic matter.
- The phenomenon of pollution is experiencing a significant surge as a result of the escalating levels of industrialization.
- Factories produce a significant volume of wastewater. Several industries, such as paper mills, food processing plants, and jute mills, generate significant volumes of wastewater.
- Various environmental factors contribute to the increase in biochemical oxygen demand (BOD).
- These factors encompass surface runoff, the presence of floating debris, the decomposition of deceased organisms and vegetation, as well as soil erosion, among others.
- There exists a limited number of chemicals that have an impact on the biochemical oxygen demand (BOD) of potable water.
- One example of a compound that can significantly elevate the biochemical oxygen demand (BOD) of water is phosphate.
- Phosphate pollution originating from American households has emerged as a significant factor influencing the levels of biochemical oxygen demand (BOD) in aquatic systems, assuming a prominent role in recent times.
In short, various sources contribute to the biological oxygen demand (BOD) in ecosystems. These sources encompass a range of organic matter, such as leaves and woody debris, deceased flora and fauna, animal excrement, effluents from industrial facilities like pulp and paper mills, wastewater treatment plants, feedlots, and food-processing plants, as well as malfunctioning septic systems and urban storm-water runoff.
How is Biochemical Oxygen Demand determined?
The determination of BOD levels can be achieved through one of two methods. The two methodologies employed in this study are empirical tests:
First method: The water sample is maintained at a consistent temperature of 20°C in a dark environment. After a period of five days, the oxygen content is assessed. The measurement of oxygen consumption during the designated time period provides insight into the water’s oxygen demand in relation to its initial value.
Second method: If it is predicted that the BOD would be extremely high or if there are other dangerous or inhibiting compounds present in the water, the sample can be diluted before it is ever taken. When this is done, the risk of there not being enough oxygen to decompose the organic matter can be reduced. Because of this, the outcome of the measurement would be inaccurate. By comparing the values from before and after the measurement, the oxygen consumption that occurred during the measurement time can now be calculated, just as it was in the first method.
After five days, a calculation is made to determine the amount of residual dissolved oxygen in the water sample. This oxygen can be used to get an estimate of the BOD level. After five days, the BOD concentration in drinking water should be considerably lower than 1 mg/l. It is needed that the BOD of the wastewater coming from the sewage treatment plant be around 20 mg/l for it to be regarded as acceptable.
Typical Values of Biochemical Oxygen Demand
- Below 1 mg/L, the water is considered to be of pristine quality.
- Water with a moderate level of pollution, between 2 and 8 mg/L.
- Above 8 mg/L, the water is considered to be extremely polluted.
Affecting Factors of Biochemical Oxygen Demand
- The amount of dissolved oxygen in rivers and streams is influenced by the biological oxygen requirement of the environment. The rate of oxygen consumption is influenced by a number of factors, including pH and temperature, as well as the types of bacteria and materials present, both organic and inorganic.
- The higher types of aquatic life require less oxygen to sustain their metabolisms. The results of having a high BOD are equivalent to the implications of having a low dissolved oxygen level.
Effect of Pollution on Biochemical Oxygen Demand
The water quality of the bodies of water is significantly deteriorating as a direct result of the increasing levels of pollution and urbanization.
- Management of water quality is critical to ensuring that ecological processes are carried out as intended.
- The output of sewage in urban areas is significantly increased as a result of urbanization. The number of sewage treatment plants that were available was insufficient for the task of processing such high volumes of sewage. It was common practice to discharge untreated sewage straight into bodies of water, which resulted in huge pollution and an increase in the BOD levels of such bodies of water. This also contributed to a rise in the prevalence of water-borne diseases such as cholera, dysentery, jaundice, and other similar conditions.
Effect of high BOD on the aquatic system
Increasing the biochemical oxygen demand (BOD) has the same effect as decreasing the amount of dissolved oxygen in the water. When there is a drastic increase in the biological oxygen demand (BOD) of a body of water, the aquatic life in that water is negatively impacted.
- Microbes are responsible for a large reduction in the amount of oxygen required by aquatic creatures for respiration and metabolism. This reduction is due to the microbes’ ability to break down organic waste.
- Fish and aquatic vegetation will perish as a direct consequence of this, as well as the entire aquatic environment will be thrown into disarray.
- Even low-oxygen organisms like catfish and carp are put in danger when the oxygen concentration drops below 5 parts per million (ppm).
- At these levels, there is just no chance of survival for freshwater fish such as catla and rohu. The natural splendor and attractiveness of the water body as a whole have been marred.
Use of Biochemical Oxygen Demand in Wastewater Treatment Facilities
- In secondary sewage treatment, also known as biological sewage treatment, biochemical oxygen demand is a factor that is considered. After the first treatment, in which the floating debris is removed by sequential filtration and sedimentation, the primary effluent is sent on to the aeration tanks, where it is constantly agitated and the air is pumped into it. This is the final step in the treatment process. There is a rapid proliferation of heterotrophic microorganisms forming flocs in aeration tanks. Bacterial colonies that are attached to fungal filaments are referred to as flocs.
- These bacteria are responsible for the breakdown of any organic stuff that may be found in the primary effluent. The BOD of the water is decreased to a more manageable level by the treatment process. Currently, we refer to this as the activated sludge. This effluent from the aeration tanks is then transferred to the settling tanks, where the bacterial flocs are allowed to settle before being treated with anaerobic microorganisms and physicochemical processes. Only then is the effluent allowed to be discharged into bodies of water.
How to reduce BOD in water?
The following strategies can be utilized to bring the water’s biological oxygen demand down to an acceptable level:
- Advanced Oxidation Processes (AOP) include the use of H2O2/ UV, O3/UV, Fenton’s reagent (H2O2+FeSO4), and other similar substances.
- The use of alum or cationic polymers in the process of coagulation
- A technique involving floating on dissolved air.
- Both flocculation (with substances like chitosan, isinglass, or polyelectrolyte) and sedimentation are processes that are required for this phenomenon.
- Reverse osmosis
- Utilizing the up-flow anaerobic sludge blanket reactor, abbreviated as UASB.
All life on Earth requires water to function properly. Therefore, it is crucial that we do our best to reduce water waste and avoid contamination. Water pollution is a direct threat to all forms of life, including humans. Increases in the biochemical oxygen demand of water have negative consequences for aquatic ecosystems and, by extension, for the health of the biosphere as a whole. Maintaining these ecosystems should be a priority of ours. All forms of life are entitled to the same share of the planet’s resources as humans. We need to control in our greed and focus instead on protecting water sources and lowering their bacterial oxygen demand (BOD).
Importance of Biochemical Oxygen Demand
- The biological oxygen demand (BOD) of a body of water is determined by the amount of oxygen that is used by microorganisms during the process of breakdown of organic waste.
- When treating sewage or wastewater, the biological oxygen demand (BOD) is computed to determine the amount of organic waste that has been broken down by aerobic bacteria.
- It provides an indication of the quantity of organic pollution that is present in an aquatic habitat.
- In soils, sewages, sediments, waste, sludge, and other types of materials, it quantifies the amount of organic matter that is present.
- In the medical and pharmaceutical industries, BOD is also employed as a test to determine the amount of oxygen that is consumed by cell cultures.
- The quality of the effluents that are discharged into the stream water may also be examined using this source, which is another function it provides.
Uses of Biochemical Oxygen Demand
- In the field of science, the self-cleaning ability of streams is measured by using something called the biological oxygen demand.
- In the field of sanitary analysis, this method is crucial for estimating the concentration of polluted water and industrial waste, as well as for analyzing sewage.
- In addition to this, it acts as a quality control checkpoint for the effluents that are discharged into the water of the stream.
Conclusion
Biochemical oxygen demand (BOD) refers to the quantity of dissolved oxygen (DO) required by aerobic biological organisms to decompose organic matter within a specified water sample, under defined temperature conditions, and over a designated duration. The biochemical oxygen demand (BOD) value is typically quantified as the amount of oxygen consumed, measured in milligrams, per liter of sample over a five-day incubation period at a temperature of 20 °C. This metric is frequently employed as an indicator of the level of organic contamination present in water. BOD reduction serves as an indicator of the efficacy of wastewater treatment facilities. The biochemical oxygen demand (BOD) of wastewater effluents is employed as an indicator of the immediate effect on the dissolved oxygen levels in the water body where they are discharged.
The analysis of biological oxygen demand (BOD) shares functional similarities with the analysis of chemical oxygen demand (COD), as both methods quantify the concentration of organic compounds present in water. Nevertheless, the analysis of chemical oxygen demand (COD) is characterized by a lower level of specificity as it encompasses the measurement of all substances that can undergo chemical oxidation, rather than solely focusing on the quantification of biologically oxidized organic matter.
References
- https://www.vedantu.com/biology/biological-oxygen-demand
- https://microbiologynote.com/biological-oxygen-demand-bod/
- https://www.vedantu.com/biology/biochemical-oxygen-demand
- https://dnr.wisconsin.gov/topic/labCert/BODanalysis
- https://byjus.com/biology/biochemical-oxygen-demand/
- https://www.watereducation.org/aquapedia-background/biochemical-oxygen-demand
- https://www.ysi.com/parameters/biochemical-oxygen-demand-bod
- https://www.usgs.gov/special-topics/water-science-school/science/biochemical-oxygen-demand-bod-and-water?qt-science_center_objects=0#qt-science_center_objects
- https://www.wwdmag.com/utility-management/article/10938701/what-is-biological-oxygen-demand-bod
