Changing states of matter is the process of turning solid into gas or liquid, and vice versa.
Whenever a substance undergoes energy absorption or dissipation, it transitions into its physical state. The underlying cause of this transformation can be attributed to the augmentation of kinetic energy. Upon assimilating the energy, the atoms or molecules within the substance commence to exhibit heightened levels of motion, thereby inducing a surge in their kinetic energy, which subsequently propels the particles to disperse over considerable distances. This process is known as the changing state of matter.
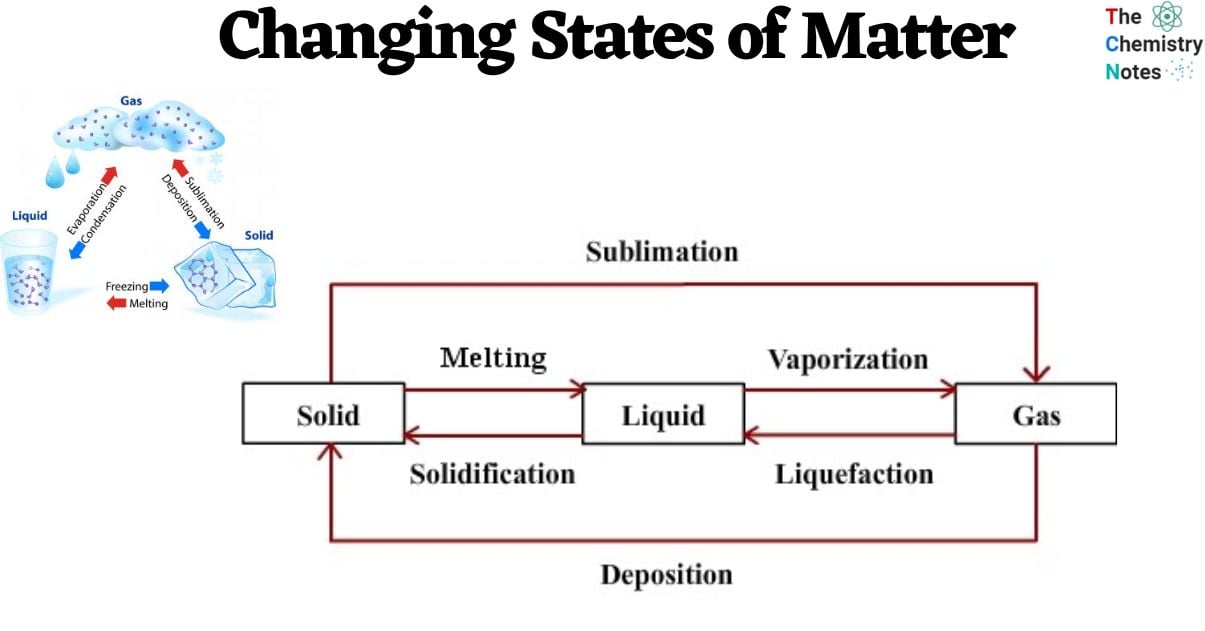
In the realm of everyday experiences, one can observe the presence of four distinct states of matter: solid, liquid, gas, and plasma. Numerous intermediate states have been identified, including liquid crystal, while certain states are exclusively observed under extreme circumstances, such as Bose-Einstein condensates (at extremely low temperatures), neutron-degenerate matter (at extremely high densities), and quark-gluon plasma (at exceedingly high energies).
Interesting Science Videos
Changes of State
A physical transformation in a substance is referred to as a modification in the state or properties of the substance. These modifications exhibit a reversible nature and do not involve any changes to the chemical composition of the essence. Furthermore, deposition, sublimation, melting, freezing, and vaporization are manifestations of state changes in matter. When discussing the phenomenon of state changes in matter, one may consider the following scenario: the transformation of ice cubes into liquid water through melting or the conversion of a liquid into vapor through boiling.
These observable alterations in the state of matter prompt the question: what is the underlying cause for these changes?
The phenomenon occurs when there is a transfer of energy to or from a substance, resulting in a modification of its state. Furthermore, when a substance acquires energy, its constituent molecules and atoms exhibit increased rates of motion, resulting in additional kinetic energy that causes the particles to move sufficiently apart from one another, thereby inducing changes in their overall shape. Typically, this form of energy is referred to as thermal energy, also known as heat. Moreover, there are numerous additional instances of transitions between different states of matter that warrant examination.
Causes of Phases Changes
The Impact of Temperature Variation on Matter
As the temperature of solids, such as ice, rises, the intermolecular forces governing particle interactions within the solid weaken, leading to augmented mobility of the particles. Consequently, the kinetic energy of the particles increases. Subsequently, the particles are liberated to traverse with enhanced velocity, culminating in a juncture wherein the solid undergoes a transformation into a liquid state. The melting point refers to the lowest temperature at which a solid substance undergoes a phase transition into a liquid state under standard atmospheric pressure.
The temperature of a solid remains constant throughout the process of melting. The phase transition of pure water or ice from a solid state to a liquid state occurs at a temperature of 273.15 K (0°C). During this transition, the temperature remains constant until all of the ice has completely transformed into liquid water. This phenomenon occurs due to the transfer of thermal energy, either gained or lost, which facilitates the transformation of ice into its liquid form, commonly known as water. The quantity of thermal energy necessary to transform 1 kilogram of solid substance into its liquid state at the temperature of its melting point is commonly referred to as the latent heat of fusion.
When additional thermal energy is introduced to the water, the intermolecular forces weaken even more, resulting in an increase in the kinetic energy of the water particles and subsequently leading to a rise in temperature. A point is reached at which the entirety of the liquid water undergoes a transformation into steam.
The boiling point refers to the lowest temperature at which a liquid undergoes a phase transition into a gaseous state under standard atmospheric pressure. The boiling point of pure water is 373.15K (100°C).
The latent heat of vaporization refers to the amount of heat energy needed to transform 1 kilogram of a liquid substance into its gaseous state at the specific temperature corresponding to its boiling point.
This is the reason why the water temperature remains constant at 373.15 K (100°C), during the process of converting it into steam.
Certain solids have the ability to undergo a direct transition into the gaseous phase when exposed to elevated temperatures and subsequently revert back to their solid state upon cooling.
Upon the application of heat, camphor undergoes a process known as sublimation, wherein it transitions directly from its solid state to its gaseous state.
When subjected to heat within a porcelain dish, camphor fragments undergo sublimation, transforming into a gaseous state. This process occurs within an apparatus consisting of an inverted funnel, which is sealed by a cotton plug.
The gaseous substance ascends and, due to the lower temperature in proximity to the porcelain dish, specifically towards the constricted aperture of the funnel, undergoes condensation and transforms into solid camphor, subsequently adhering to the inner surfaces of the funnel.
The Impact of Pressure Variation on Matter
Upon opening a soda canister, the phenomenon of bubbles ascending in the water can be observed. The formation of these bubbles can be attributed to the existence of carbon dioxide gas within the water.
While carbon dioxide gas is typically considered insoluble in water, it can, however, be dissolved in water under extremely high pressure. The impact of pressure variation on solids and liquids is typically negligible. However, in the context of gases, an increase in pressure leads to a decrease in volume.
In a gaseous state, the constituent particles are widely dispersed within a specified volume. Nevertheless, under the influence of external pressure, the gas particles undergo a reduction in intermolecular distance, resulting in a corresponding decrease in the gas volume.
This process results in the transformation of a gaseous substance into a liquid state. In an analogous fashion, carbon dioxide gas has the potential to undergo a transformation into solid carbon dioxide, commonly referred to as dry ice, given specific circumstances characterized by elevated pressure levels of approximately 5 atm and reduced temperatures of -56°C.
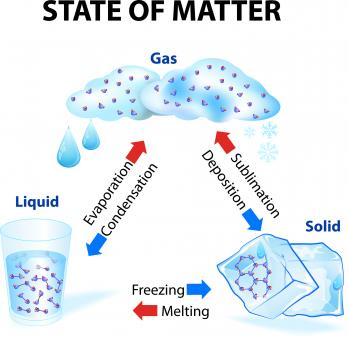
Changes of State Between Liquid and Solid
Let us take a look at the scenario where a tray is used to make ice cubes in refrigerators.
Freezing
- The Thermal energy is transferred from the tray to the lower-temperature ambient air within the freezer. Subsequently, the heated liquid within the freezer dissipates its thermal energy into the frigid ambient air.
- This process of heat transfer persists until the water particles have dissipated all of their kinetic energy. The individuals are compelled to remain in stable locations due to the formidable force of attraction between them.
- The liquid undergoes a phase transition and is transformed into a solid state, as exemplified by the conversion of water into ice through this particular process.
- Accordingly, freezing is characterized as the phenomenon wherein a substance transitions from a liquid state to a solid state. Furthermore, the freezing point can be defined as the critical temperature at which this transition occurs, leading to the solidification of the substance.
Melting
- If the ice cubes were removed from the freezer and exposed to a heated environment, they would undergo a process of energy absorption from the surrounding warmer air.
- The assimilated energy would empower them to surmount the force of attraction that binds them, thereby allowing them to disengage from their stationary state as ice.
- The phenomenon by which solid substances undergo a transition into a liquid state is referred to as melting.
- The melting point refers to the specific temperature at which the solid state of a substance undergoes a phase transition, resulting in its transformation into a liquid state.
Changes of State Between Liquid and Gases
When thermal energy is transferred from the cooktop to the utensil, the water contained within the bowl undergoes an increase in temperature. Subsequently, the water assimilates the thermal energy. We will take a look at the changes in the state.
Vaporization
- When water is subjected to a sufficient amount of thermal energy, it undergoes a phase transition and enters a state of boiling once it reaches its boiling point. Subsequently, the phenomenon of bubble formation occurs within the vigorously boiling water.
- This phenomenon occurs when the water particles acquire a sufficient amount of energy to surpass the intermolecular forces between them and transition into the gaseous phase.
- The formation of bubbles occurs within the water, which is subsequently released in the form of steam, while the dissipation of heat transpires from the bowl.
- Nevertheless, vaporization is precisely characterized as the process by which a substance transitions from its liquid state to a gaseous state.
- Furthermore, the boiling point of a liquid refers to the specific temperature threshold at which it undergoes the boiling process.
Condensation
- Whenever an individual takes a shower using hot water within an enclosed washroom, it becomes evident that condensation accumulates on the mirror surface within the said washroom.
- This phenomenon occurs due to the process of evaporation, wherein water undergoes a phase transition from its liquid state to a gaseous state. When the water vapor comes into contact with a cold surface, it releases its thermal energy and subsequently experiences a decrease in temperature.
- This cooling effect is analogous to the behavior observed in a mirror found in a restroom. Hence, condensation is the phenomenon by which a gaseous substance undergoes a transformation into its liquid state.
Changes of State Between Solid and Gas
When undergoing a phase transition from solid to gas, it is necessary for the substance to first switch into the liquid phase before undergoing evaporation and subsequently transforming into the gaseous state. However, under certain circumstances, a solid substance may undergo a direct transformation into its gaseous state. Likewise, the gaseous state can undergo direct conversion into a solid state.
Sublimation
- Sublimation is the transformative process whereby a solid substance undergoes direct conversion into its gaseous state.
- When a solid assimilates a sufficient quantity of energy, the intermolecular forces binding its constituent particles are completely nullified.
- The phenomenon of sublimation occurs when a solid substance, such as dry ice, undergoes heating.
- Air fresheners are also present in the context of sublimation, such as those commonly employed in restroom facilities.
Deposition
- The deposition process refers to the transformation of a substance from a gaseous state directly to a solid state, bypassing the intermediate liquid state.
- The laws of thermodynamics govern the process of deposition.
- Moreover, the process of deposition can be considered the antithesis of the sublimation process.
Video on Changing States of Matter
References
- https://www.twinkl.com/teaching-wiki/states-of-matter
- https://byjus.com/physics/changing-states-of-matter/
- https://testbook.com/physics/changing-states-of-matter
- https://www.geeksforgeeks.org/change-of-state-of-matter/
- https://www.vedantu.com/physics/changing-states-of-matter
- https://www.studysmarter.co.uk/explanations/physics/particle-model-of-matter/changes-of-state/

