Interesting Science Videos
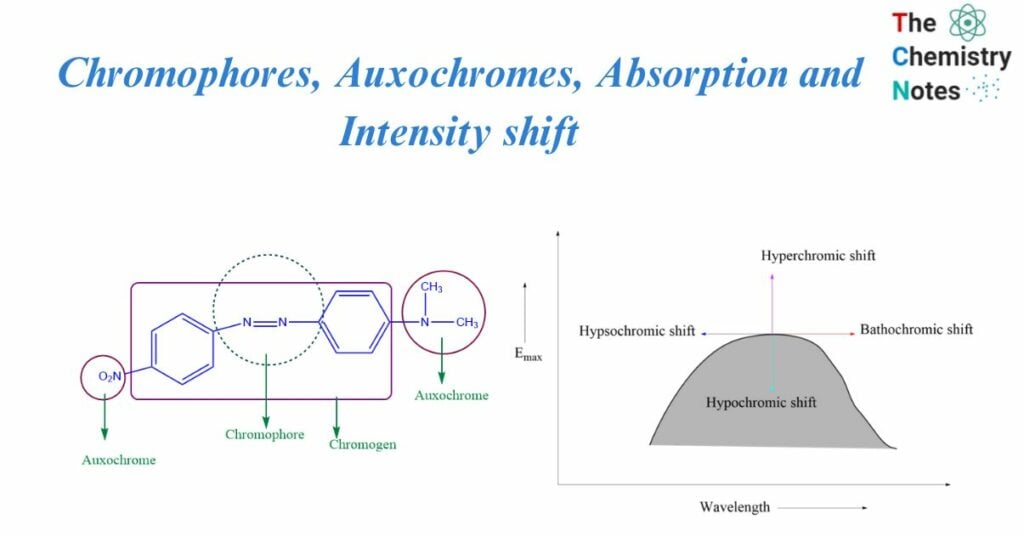
Chromophores
It’s a Greek term i.e., Chroma “colour” and phoros “bearer”. It is defined as any isolated covalently bound group that exhibits distinctive electromagnetic radiation absorption in the UV or visible area. The chromophore-containing compound is chromagen. The electron is loosely bonded in -N=N-. These loosely bound electrons required less energy for electronic transition, and the absorption band occurred in the near ultraviolet region.
Other example of chromophores includes C=C, C=O, and NO2
A chromophore is an atom or group that contributes to the color of a chemical. Molecules absorb some visible spectrum wavelengths while reflecting others. The wavelength reflected by the molecule is what we experience as color.
The energy difference between two distinct chemical orbitals in a chromophore falls within the visible spectrum region. Then, the chromophore was exposed to the visual spectrum, which activated the electrons from their ground state. As a result, when exposed to light, a chromophore changes conformation.
If more than one chromophore is required to impart the color in a compound then it is known as dependent chromophores. For example: Acetone having one ketone group is colorless while diacetyl having two ketone groups is yellow.
Different kinds of chromophores
There are two kinds of chromophores:
- Chromophores that can only π contain electrons. They only go through π -π * transitions, such as the ethylenic group (C=C) and the acetylenic group (C C).
- Chromophores that contain π as well as n (nonbonding) electrons. This chromophore has a single pair of electrons. As a result, they are responsible for two types of transitions, namely n-π * and π -π *, such as nitro group (-NO2), azo group (-N=N-), nitro group (-NO3), carbonyl group (>C=O), and nitrite group (-ONO).
Auxochromes
They are saturated and unsaturated groups that consist of one or more non-bonded electron pairs. This group, which is linked to the chromophore, assists in wavelength variation by raising the intensity of absorption and increasing max.
It is a group that does not behave as a chromophore on its own, but when linked to a chromophore, it shifts the adsorption towards longer wavelengths while increasing the intensity of adsorption. Auxochrome enhances a molecule’s color, whereas chromophore imparts color to a molecule. So, Auxochromes are a colour-enhancing group.
Examples of Auxochromes include OH, NH2, NHR, and NR2
Absorption and Intensity Shifts
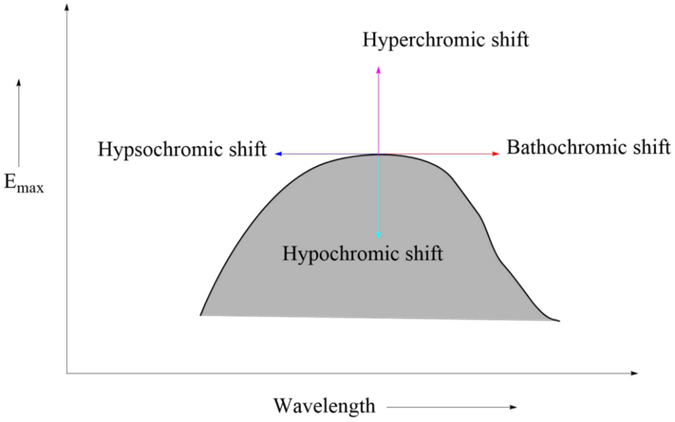
Bathochromic shift
It is an effect by which the absorption maximum is shifted towards a longer wavelength due to the presence of an autochrome or by a change of solvent. Bathochromic shifts are also called redshifts.
Hypsochromic shifts
This is the effect by which the absorption maximum is shifted towards a shorter wavelength. It is also called the blue shift. It may be caused by the removal of conjugation and changing the solvent’s polarity.
Hyperchromic effect
It is an effect due to which the intensity of absorption maximum increases Emax increases. B-band for pyridine at 275 mμ, Emax 2750 is shifted to 262mμ, Emax 3560 for 2-methyl pyridine. The introduction of autochrome usually increases the intensity of absorption.
Hypochromic effect
It is defined as an effect due to which the intensity of absorption maximum decreases, i.e., extinction coefficient Emax decreases. The introduction of the group which distorts the geometry of the molecule causes the hypochromic effect.
Factors influencing absorption and wavelength change
Effect of conjugation
According to MOT, as the number of pi electrons increases, so does delocalization. Because of this rise in delocalization, the molecules in the sample become stabilized and attain a lower energy state. This decrease in energy causes a shift in wavelength towards a higher wavelength, which is known as redshift.
Due to double bond conjugation, 1,3-butadiene absorbs at a higher wavelength, 217 nm, while 1,2-butadiene absorbs at 210 nm.
Presence of aromatic ring
The aromatic ring, particularly when two or more rings are conjugated (polycyclic compounds), absorbs a greater wavelength in the visible range, altering the absorption spectra. For example, Naphthalene absorbs at 268nm while anthracene absorbs at 311nm.
The presence of auxochrome
shifts the adsorption towards longer wavelengths while increasing the intensity of adsorption.
Solvent polarity
Polarity has a significant impact on the position and strength of absorption bands. The increase is related to the n- -π* and π – -π* transitions.
In the presence of a polar hydrolytic solvent (such as water), hydrogen bonds form with the autochrome’s lone pair of electrons. As a result, the autochrome’s energy decreases to an amount equivalent to the bond formation energy, and the energy difference between HOMO and LUMO increases, resulting in a hypsochromic shift for the n- -π* transition.
While the π* orbital is more polar than the π orbital for the π- π* transition, it is stabilized to a greater extent in the presence of a polar solvent. Because the energy gap between π- π* is lowered due to the stability of the π* orbital, this results in a bathochromic shift.

Helpful informaion