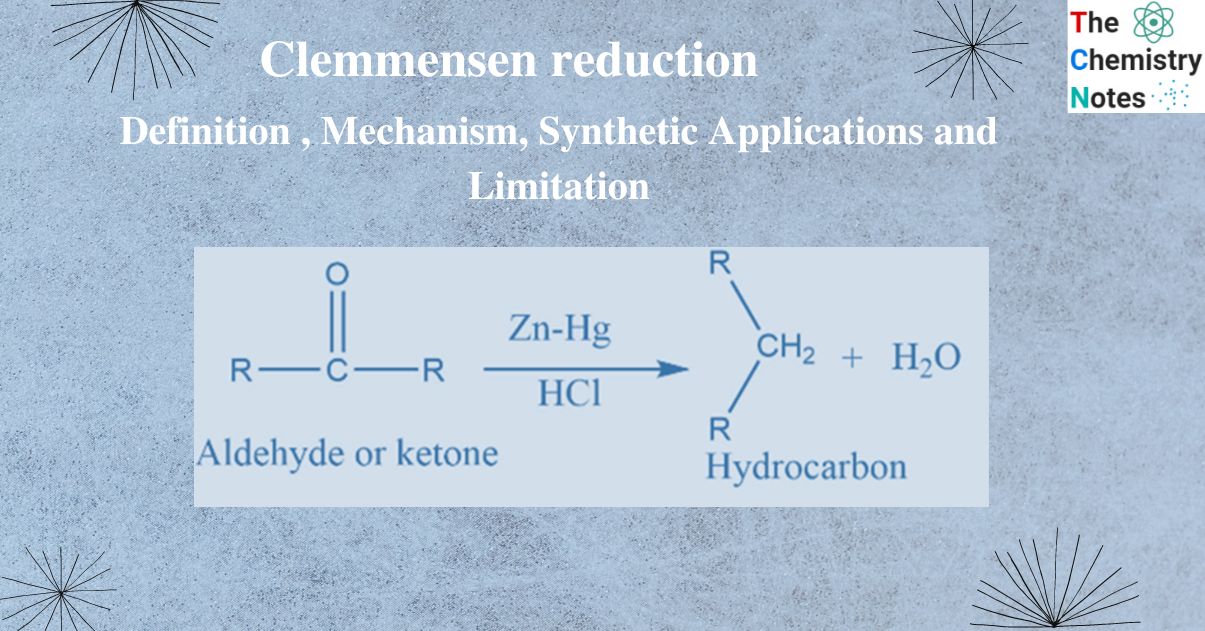
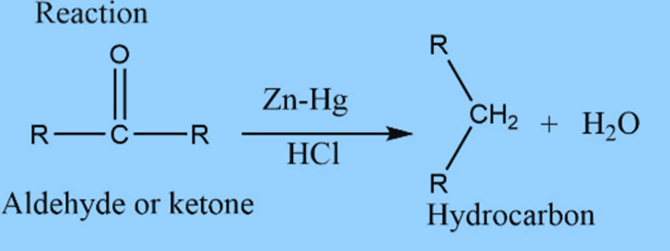
Clemmensen reduction involves the reduction of aldehyde or ketone into alkane.
Aldehyde or ketone reacts with Zn-Hg in the presence of conc. hydrochloric acid to form the corresponding hydrocarbon. This reaction is known as Clemmensen reduction.
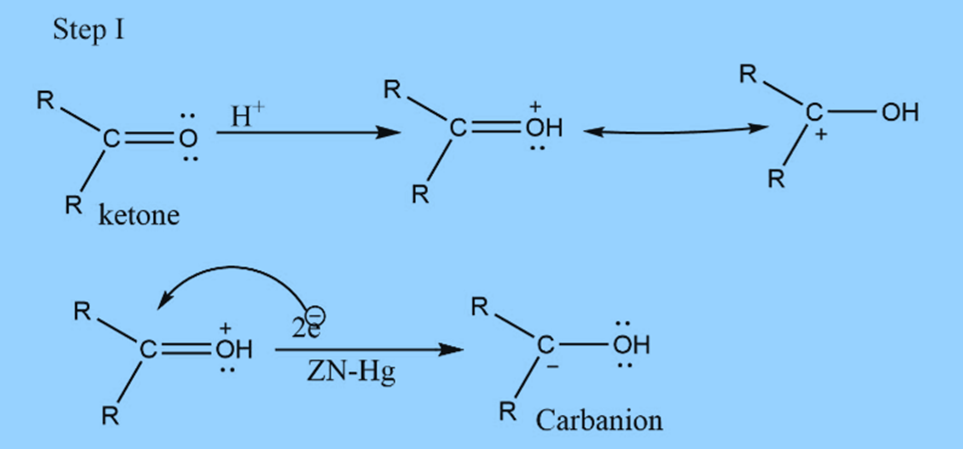
In this reaction, acid supplies proton to the oxygen. At the same time, metal supplies an electron pair to electron-deficient carbonyl carbon to form carbanion.
The carbanion mechanism or the carbenoid mechanism can both be used to carry out this reaction mechanism. The carbanion mechanism entails a direct Zn attack on protonated carbon, whereas the carbenoid mechanism is a radical reaction that involves a reduction at the zinc catalyst surface.
Interesting Science Videos
Mechanism of Clemmensen reduction
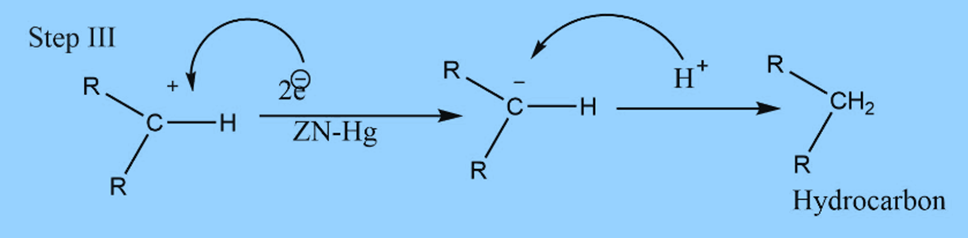
The mechanism involves three steps
First step : Formation of carbanion
Second step : Elimination of water
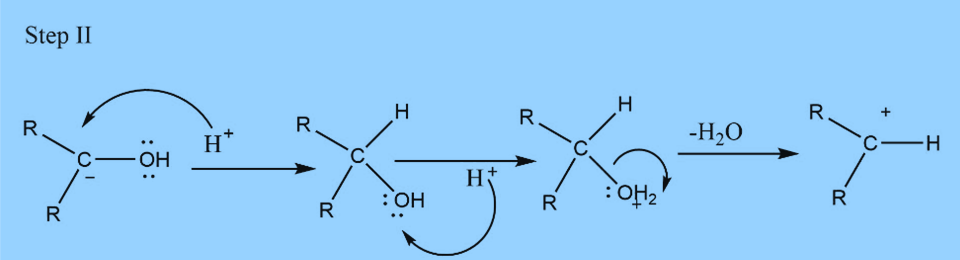
Third step: Addition of proton
Apllications of Clemmensen reduction
- The reduction mechanism is commonly used to convert the carbonyl group to the methyl group.
- It is an important application in preparing polycyclic aromatic compounds containing unbranched side hydrocarbon chains.
- It is commonly used to convert acyl benzene to alkyl benzene.
Some synthetic applications
1. Conversion of acetophenone into ethyl benzene.

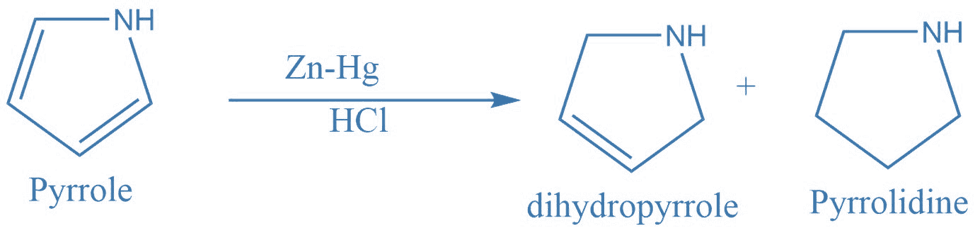
2. Conversion of pyrrole to dihydropyrrole and pyrrolidine
Limitations
This reaction can not be used for acid senstitive substances. Additionally, this method cannot reduce the -COOH group.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
