Studies of the kinetics of a chemical system, like the effect of changes in reactant concentrations, can be used to figure out things that happen on a microscopic scale. These kinds of studies led to the collision model of chemical kinetics, which is a good way to understand how chemical species behave when they react. The collision theory explains why chemical reactions usually happen faster when the temperature is higher.
Interesting Science Videos
What is Collision Theory?
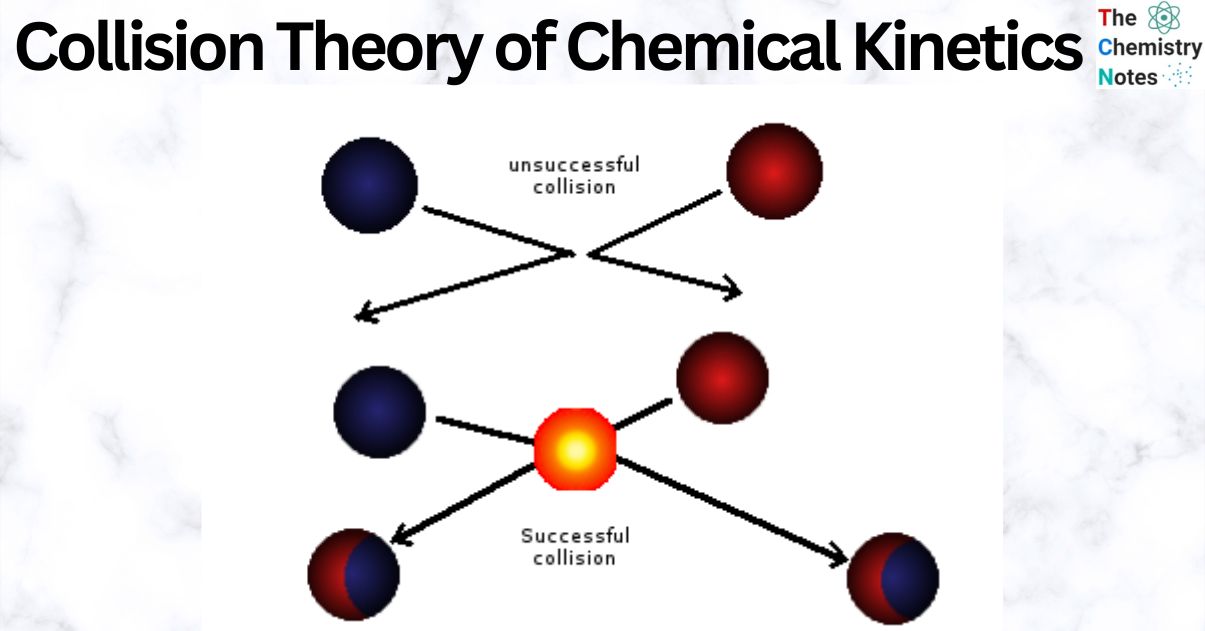
According to the collision theory, the rate of a chemical reaction is directly related to the number of collisions between the molecules of the reactants. The collision theory in chemistry is a simple way to explain what starts chemical reactions.
Theoretically, the rate of reaction gets faster as the molecules of the reactants bump into each other more. In reality, though, only a small number of collisions are what we call “effective collisions,” which are the only kind of collision that creates a product.
The collision frequency is defined as the average number of collisions that occur in a given amount of time and space inside the reacting mixture. The letter Z is often used to denote this symbol. This is an example of a bimolecular elementary reaction:
P + Q → Product
In accordance with collision theory, the previous reaction rate is defined as
Rate = ZPQρe−Ea/RT
Where:
ZPQ = collision frequency of reactants P and Q
Ea = Activation Energy
R = Universal Gas Constant
T = Absolute Temperature
ρ = is the steric factor
Another parameter with significant influence on the rates of chemical reactions is the activation energy (Ea). Arrhenius used the term “activation energy” to characterize the minimal energy input required by reactants in a chemical reaction.
When two molecules meet with energies higher than or equal to the activation energy, the Arrhenius Equation predicts that products will be formed. This was not the case with every response, either. There was a great deal of variation in reactions involving complex chemicals. This is because not all molecules with sufficient energy (activation energy) collided. Only a small fraction of them actually collided in ways that generated useful byproducts.
The researchers found that the molecules’ kinetic energy is not the only element in determining the outcome of the reaction. Only molecules that are properly oriented and have a high enough threshold energy (activation energy) during the collision can yield products, the researchers observed. They calculated the probability factor P to account for effective collisions.
In brief, an effective collision, leading to the formation of products, is conditioned by the activation energy and proper orientation of the interacting molecules. The activation energy and the effective collision regulate the response rate in collision theory.
Postulates of Collision Theory
Collision theory is based on following postulates:
- In order to react, molecules must collide.
- The rate of reaction is proportional to the rate of reactant collision molecules. When the number of collisions between reactants increases, the rate (or speed) of the reaction does as well.
Rate of Reactions ∝ Number of Collision per Time
- Molecules colliding with one another must have enough energy to cause bond disruptions in order to start a reaction.
- When the temperature rises, the molecules will accelerate and clash more forcefully, considerably increasing the possibility of bond cleavages and rearrangements.
- In order for neutral molecules to participate in a reaction, they must first obtain the activation energy necessary to stretch, bend, or distort one or more bonds.
Types of Collision
Elastic Collision: An elastic collision is one in which both the kinetic and momentum energies of the system are preserved. When different subatomic particles collide, they mostly just stretch out elastically. For instance, the collision between two balls made of glass or steel is usually elastic. Conservative forces are exerted during inelastic collisions.
Inelastic Collision: In an inelastic collision, only momentum is maintained and the kinetic energy of the colliding objects is lost. Many inelastic collisions occur every day in our lives.
Theoretical Terms Related with the Collision Theory
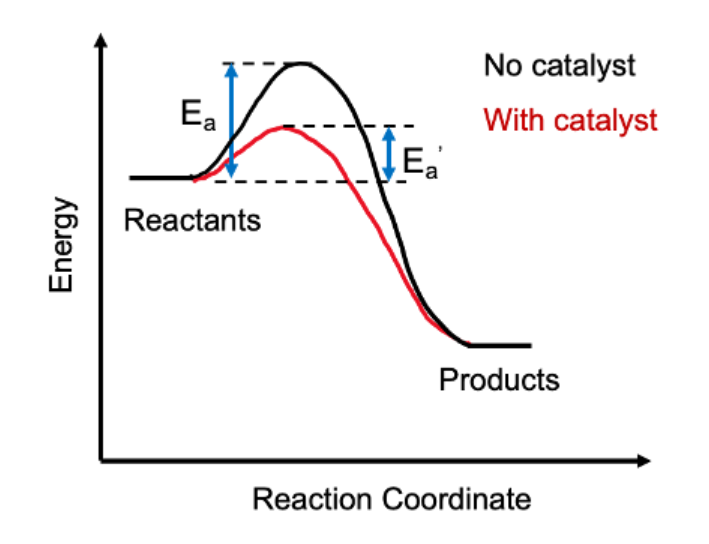
Catalyst: A chemical that does not react yet reduces activation energy and speeds up a process.
Kinetic Energy: Energy associated with motion is known as kinetic energy.
Activation Energy: The minimal amount of energy that a reactant must have to react.
Kinetics: It is the study of the rate and speed of reactions.
Reaction Rate: The speed at which a process takes place is known as its reaction rate.
Fundamental Principles of Collision Theory
There are two fundamental ideas in collision theory:
- Orientation
- Energy
Orientation: To collide, molecules must first come together in the proper direction. Consider the interaction of ethene with hydrogen bromide. Bromoethane results from this. The hydrogen atom attaches to the C=C double bond during the process. This requires a close approach and collision of the hydrogen end of the hydrogen bromide molecule with the double bond in ethene. Nothing will happen if the hydrogen atom strikes one of the carbon atoms or the C-H single bonds rather than the C=C double bond, or if the bromine atom strikes the double bond.
Energy: Molecular collisions require sufficient energy for them to respond. This is so because the initial step in all reactions is the breaking of bonds, which is an endothermic process that requires energy. The activation energy is the quantity of energy required in a reaction, which varies based on the species involved and the reaction itself.
The minimal quantity of energy required to initiate a chemical reaction is called activation energy. It is frequently expressed in kJ mol-1 and has the symbol Ea.
According to collision theory, molecules will only respond if they reach or surpass the activation energy, even if they collide with the ideal orientation. They will just bounce off of one other if they are not sufficiently energetic.
Activation Energy
The minimum amount of energy needed by the reacting particles in a particular reaction for that reaction to take place is known as the activation energy. Unless there is sufficient energy in the collision for the activation energy to be produced, particles do not respond. Energy must be provided to activate a reaction before it can take place. Chemical bonds in the reactants must be disrupted, which requires energy, for a chemical reaction to start. Activation energy is the quantity of energy needed to start the reaction. The reaction can start at room temperature without being heated when the activation energy is low enough.
Example of Collision Theory
Let’s use a common, non-chemical example—a vehicle crash—to picture what happens in collision theory. When cars bump into each other, it doesn’t always end in a major accident. Although though both involve automobile crashes, a major motorway accident and a little fender bender in a parking lot have distinct differences. The direction of the cars and the force of the crash are what set a wreck apart from other types of collisions. When compared to being struck in the fender or bumper area, vehicles are more likely to crash when struck on the side. A crash is more likely to occur if the cars contact with more force.
Pool balls also known as billiard balls provide another tangible illustration of collision theory. The particles of air in the container clash in the same way that billiard balls do. To send a billiard ball into the pocket, the cue ball must make a clean contact with it at the right angle and with enough force. Many cue ball–billiard ball collisions will merely result in the balls bouncing off each other if you are not a skilled pool player. But, if the cue ball has enough speed and direction, it can successfully collide with a billiard ball, sending it into the pocket.
To enhance the frequency with which the cue ball collides with other balls, hence increasing the number of balls sent into the pockets, we must either improve our aim and the power with which we strike the cue ball, or we must employ more cue balls. There are no billiard balls or pockets in a chemical reaction, but the concepts are comparable. In reality, the behavior of gas particles in a collision is very similar to that of billiard balls.
Factors Affecting Collision Rate
The number of particles or molecules per unit of space: The more particles there are in a particular volume, the more frequently they will collide with one another.
Pressure/volume: Gaseous particles, which occupy the greatest space, have a collision rate that is sensitive to changes in pressure and volume. As pressure (or volume) is raised, collision frequency rises.
Temperature: The level of heat energy in a system is a function of its temperature. Particles travel more quickly and with greater kinetic energy when the temperature is higher. As a result, there will be more collisions between them.
References
- https://study.com/academy/lesson/collision-theory-definition-significance.html
- https://www.toppr.com/guides/chemistry/chemical-kinetics/collision-theory-of-chemical-reactions/
- https://chemistrytalk.org/collision-theory-chemistry/
- https://www.inspiritvr.com/general-chemistry/kinetics/collision-theory-study-guide
- https://georgiasouthern.libguides.com/c.php?g=943952&p=6804604
- savemyexams.co.uk/igcse/chemistry/cie/23/revision-notes/6-chemical-reactions/6-1-chemical-change–rate-of-reaction/6-1-3-collision-theory/
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/collision-theory/
- Steinfeld J.I., Francisco J.S. and Hase W.L. Chemical Kinetics and Dynamics (2nd ed., Prentice-Hall 1999) ISBN 0-13-737123-3
- Laidler, K.J. Chemical Kinetics (3rd ed., Harper and Row 1987) ISBN 0-06-043862-2
