Complexometric titration also referred to as Chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex.
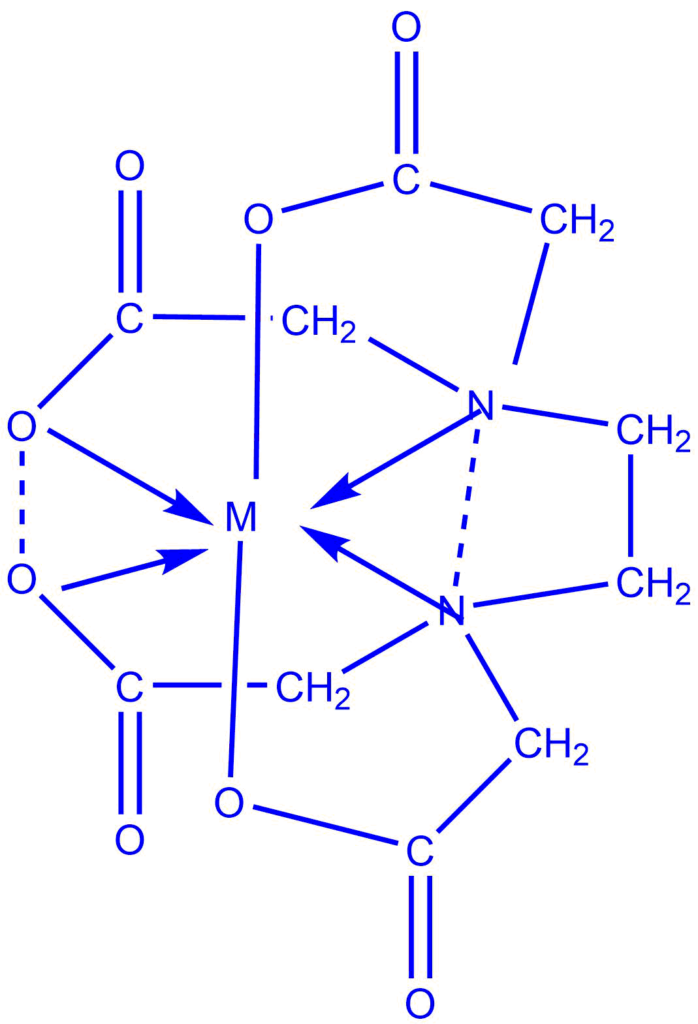
This is the titration between the metal ion and the complexing agent when an appropriate indicator is present. The indicator is a complexing agent as well, but it should combine with metal ions to produce a less stable complex and impart different unique colors during complexation and decomplexation.
A mixture of various metal ions in a solution can be determined with the help of complexometric titrations. The end-point of the titration is typically identified using an indicator that can cause an unambiguous color shift.
Interesting Science Videos
Conditions /requirements for complexometric titration
- The reaction must be quick and reversible.
- There should be no interfering situations. For instance, more than one complex should not be present during the titration when multiple complexes of various metal ions are being formed progressively with the complexing agent.
- A complexometric indicator should be capable of locating the equivalent point with reasonable precision.
Common organic reagents used in the complexometric analysis
The term “organic precipitant” refers to the organic reagents that are employed in the complexometric titration method to produce various colored complexes with the metal ions in the solution.
Examples: DMG, EDTA, Cupferron, etc.
Uses of organic precipitant
- It is used to separate the ion from the mixture.
- It forms the colored precipitation.
- It should be specific i.e, it should give a precipitate with the specific metal ion.
- Sometimes ppt can be converted to the oxides of metal.
Criteria for organic reagent (precipitant) for use in the inorganic analysis
The following are the parameters to be considered in choosing an organic reagent in quantitative analysis:
- Organic reagents must be simple to acquire, purify, dry, and preserve in a pure state.
- It must remain unchanged in the air while drying and weighing.
- Its molecular weight should be high.
- It should be easily soluble under the conditions in which it is used.
- At the equilibrium point, there must be a change in some of the solution’s physical and chemical characteristics.
- There must be a simple reaction that can be represented using a chemical equation.
- The endpoint should be clearly specified.
- It should be specific i.e., should give ppt with only one ion in the mixture of ions.
Titration of the mixture
When the mixture contains more than one metal ion then titration of such solution is carried out by different methods they are as follows:
1. By suitable control of the pH of the solution
This method is used when different metal-EDTA complexes have different stability at different pH and titration is carried out by using different indicators.
Example: A mixture of lead and bismuth can be titrated by this method.
In this method, the first titration is with bismuth at pH = 2 with xylenol indicator and then hexamine is used to raise the pH to 5 and lead is titrated.
Similar to this, titrations of bismuth and thorium can be performed in an acidic solution with a pH of 2 and an indicator such as xylenol orange or methyl thymol blue, where most divalent cations are not disruptive.
2. Use of Masking agent
Masking may be defined as the process in which a substance, without physical separation of it or its reaction products, is so transformed that it does not enter into a particular reaction. The cyanide ion serves as a powerful masking agent, forming stable cyanide complexes with the cations of Cd, Zn, Hg(II), Cu, Co, Ni, Ag, and platinum metals. It does not form complex with the alkaline earth, manganese, and lead. Therefore, by masking with an excess of potassium or sodium cyanide, it is feasible to identify cations like Ca2+, Mg2+, Pb2+, and Mn2+.
Demasking is the process in which the masked substance regains its ability to enter into a particular reaction. For example, Fluoride (F-) ion can be used to demask a mixture of Mg2+, and Mn2+.
Example: Mixture of Mg2+, and Mn2+.
First, a metal-indicator complex is formed by allowing the ion mixture to react with enough indication. EDTA solution is used to titrate it. Metal-EDTA complexes are formed when both metals react with EDTA. The Mg2+ is then demasked with F-ion and the combination is titrated with standard metal ion solution Mn2+. The amount of Mg2+ is estimated from the back titration. The amount of Mg2+ initially present may now be determined using the total EDTA used and the volume of metal ion used in the back titration.
3. By separation
If the ions are not tedious, this procedure is used. Metal first forms ppt with a complexing agent that is dissolved in the solution. The cation is then complexometrically titrated.
4. Kinetic masking
Some metal ion does not effectively enter into the complexation reaction because of its’s kinetic inertness. In this case, kinetic masking is used.
Example: The ability to titrate other metal ions without interfering with Cr (III) is made possible by the slow reaction of Cr (III) with EDTA (III).
Applications of complexometric titration
- To determine the metal content in medicines, it is commonly employed in the pharmaceutical industry.
- Many cosmetic items contain titanium dioxide. Complexometric titration can be used to analyze this.
- It is employed in the analysis of urine samples.
- It has many applications in analytical chemistry.
Advantages of complexometric titrations
- This is a simple method that doesn’t require special knowledge and abilities.
- complexometric titration is a rapid and precise method.
- The identification of mixtures of various metal ions in solution is a major benefit of complexometric titrations.
- Since it doesn’t require the use of costly chemicals, equipment, or glassware, it is a cost-effective procedure.
Disadvantages of complexometric titration
- It is a destructive procedure that frequently employs quite significant amounts of the substance under investigation.
- Since this is an open system, variables like humidity, pH, and temperature may have an impact on the outcomes.
- This can generate a significant amount of chemical waste that must be disposed of.
- This necessitates reactions taking place in a liquid phase, which is often undesired due to the chemical of interest.
Suggested video
References
- https://byjus.com/chemistry/complexometric-titration/
- https://www.vedantu.com/chemistry/complexometric-titration
- https://chem.libretexts.org/Ancillary_Materials/Demos_Techniques_and_Experiments/General_Lab_Techniques/Titration/Complexation_Titration
- https://psiberg.com/complexometric-titrations/#:~:text=Major%20advantage%20of%20complexometric%20titrations,also%20be%20performed%20using%20instruments.
- https://collegedunia.com/exams/complexometric-titration-chemistry-articleid-6319
- https://webstor.srmist.edu.in/web_assets/srm_mainsite/files/downloads/Complexometric_Titration.pdf
