Connective tissue is the most abundant and widely distributed tissue in the body, connecting epithelium to the rest of the body and providing structure. “Connective tissue” is a term given to several body tissues that connect, support, and help bind other tissues. While some connective tissues are specialized ( bone, blood), all organs have some amount of connective tissue in them which holds their parenchyma together
Interesting Science Videos
Characteristics of connective tissue
– More matrix than cells
-Connective tissues are derived from mesoderm in embryo
-mesenchyme and mucous connective tissue
-Connective tissues are vascularized
-Connective tissues can replicate (healing and repair)
-Connective tissue is not present on free surfaces or body cavities
-Connective tissue is innervated.
Components of connective tissue
• Connective tissues differ widely but are still made up of three fundamental components :
– Cells
– Protein fibers
– Ground substance
• Protein fibers and ground substance are collectively called extracellular matrix.
Extracellular Matrix of connective tissue
-The major protein component of the Extracellular Matrix of connective tissue includes collagen, elastin, fibrillin, fibronectin, laminin, and proteoglycan
1. Ground substance
- component of a connective tissue between the cells and fibers.
- The ground substance may be viscous (as in blood), semisolid (as in cartilage), or solid (as in bone).
- supports cells, binds them together, stores water, and provides a medium for the exchange of substances between the blood and cells.
- plays an active role in how tissues develop, migrate, proliferate, and change shape.
- It primarily consists of protein and carbohydrate molecules and variable amounts of water.
- Protein and carbohydrates are mainly present in the form of proteoglycans and glycoproteins.
-Proteoglycan = Protein core + glycosaminglycan
-Glycoprotein= Protein + oligosaccharide
-Also present in the ground substance are adhesion proteins, which are responsible for linking components of the ground substance to one another and to the surfaces of cells. E.g. fibronectin which binds collagen fiber and ground substance together.
2. Protein fibers
The major protein fibers are
-Collagen fiber
-Elastic fiber
-Reticular fiber
Collagen
-most abundant protein in mammals
-comprises approximately one-third of the total body protein
-collagen forms about 90% of the organic matrix of bones, 85% of tendons, 70%of skin, and 4% of liver.
Functions of collagen
-collagen gives strength, support, and shape to the tissues (high tensile strength breaks a collagen fiber of 1 mm in diameter, a load of 104O kg is needed)
-Collagen contributes to the proper alignment of cells, which in turn helps in cell proliferation, and their differentiation to different tissues and organs
-Collagen (that is exposed in blood vessels) contributes to thrombus formation.
Types of Collagen
-In humans, at least 19 different types of collagens, composed of 30 distinct polypeptide chains have been identified
-they are numbered in Romans from I to XIX
-The different types of collagen are suited to perform specialized functions in tissues
A.Fibrillar collagens – e.g. types I, II, III, V
•Typical collagens forming fibrils
B.Collagens associated with collagen fibrils –
-for example types VI, IX, XII, XIV, XVI
• Triple helix is interrupted by sections making possible the bending of the molecule.
• These collagens attach to the surface of collagen fibrils join them together and connect them to other constituents of the extracellular matrix
C.Net forming collagens – types IV, VIII and X
• Do not form typical fibrils
• Net-like arrangement
• Nonhelical globular domains on the ends of the molecule
Some fibrilar collagens
Type Molecular structure Occurrence
I [ α 1(I)] 2 [ α 2(I)] widely present, skin, vessels, tendons, gingiva, bone, cement,
dentin, periodontal ligaments
II [ α 1(II)] 3 cartilage, vitreous body
III [ α 1(III)] 3 skin, vessels, lungs, gingiva, cement, dentin, PDL
IV [ α 1(IV)] 2 [ α 2(IV)] basal membranes, formation of two-dimensional net gingiva,
periodontal ligaments
V [ α 1(V)] 3, [ α 1(V)2 α 2(V)] skin, smooth muscle, bone, cement, dentin
VI [ α1(VI) α 2(VI) α 3(VI)] laterally associated with collagen type II, widely present,
bone, gingiva, cement, periodontal ligaments
IX [ α1(IX) α 2(IX) α 3(IX)] laterally associated with collagen type II, cartilage, vitreous
body, periodontal ligaments
XII [ α1(XII)] 3 associated with collagen type I in soft tissues, PDL
Structure of collagen
-In principle, all types of collagen are triple helical structures
-The triple helix may occur throughout the molecule, or only a part of the molecule.
-composed of three similar polypeptide chains twisted around each other to form a rod-like molecule of 1.4 nm diameter, and about 300 nm length
-The amino acid composition of collagen is unique. Approximately 1/3rd of the amino acids are contributed by glycine i.e. every third amino acid in collagen is glycine. thus the repetitive amino acid sequence of collagen is represented by (GIy-X-Y)n, where X and Y represent other amino acids
– collagen may be regarded as a polymer of glycine-led tripeptide. Among the other amino acids, proline and hydroxyproline are present in large quantities
-The triple helical structure of collagen is stabilized by an extensive network of hydrogen bonds, covalent cross-links, electrostatic and hydrophobic interactions, and van der Waals forces
-The triple helical molecules of collagen assemble and form elongated fibrils, and then rod-like fibers in the tissues.
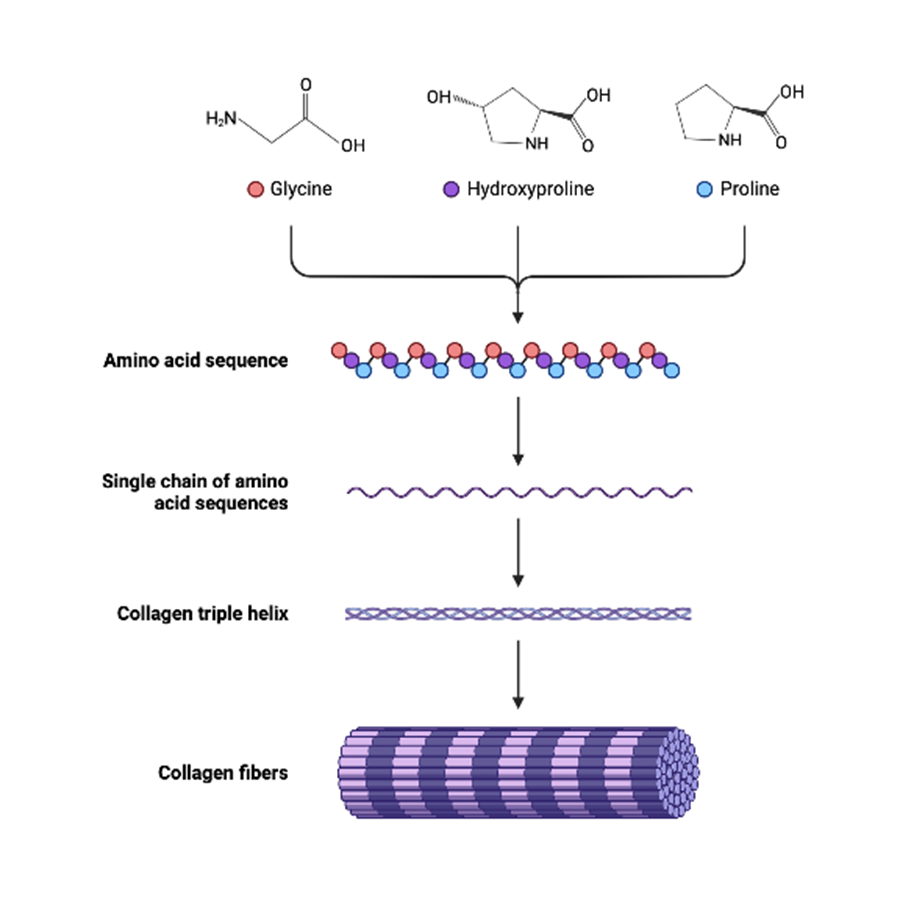
Image source: https://doi.org/10.3390/ma14092096
Biosynthesis
-occurs in cells like fibroblast, osteoblast, chondroblast, odontoblast
-synthesized in ribosome
– Collagen is an example of a protein, whose synthesis is connected with many posttranslational modifications (treatment of the polypeptide chain), which take part intra- and extracellularly.
Precursor i.e pre procollagen (containing single peptide)—directs the protein to reach endoplasmic reticulum—single peptide cleaved to form procollagen—undergoes posttranslational modification s (hydroxylation and glycosylation) and disulfide bonds formation—secreted to extracellular matrix—due to action of amino proteinase and carboxyproteinase — remove the terminal amino acid—Assembly of peptide chain to form triple helical structure.
Synthesis and posttranslational modifications of collagen
Synthesis of polypeptide chain>> Hydroxylation of proline and some lysine residues >>Glycosylation of selected hydroxylysine residues >>Formation of –S-S- bonds in extension peptides >>Triple helix formation >>Secretion of procollagen >> Proteolytic removal of propeptides >>Assembling of collagen fibrils>> Formation of cross-links
Collagen fibers
• made up of collagen.
• Collagen fibers are tough and only slightly elastic.
• They often occur in bundles with the fibers parallel to one another, which gives great tensile strength.
Abnormalities associated with collagen
As the biosynthesis of collagen is a complex process involving 30 genes and 8 posttranslational modifications y, many inherited diseases due to gene mutations, linked with collagen formation have been identified.
- Increased collagen synthesis –
• Fibrosis
• hepatic cirrhosis
• pulmonary fibrosis
• atherosclerosis
- Decreased collagen synthesis
• Genetically conditioned
– Ehlers-Danlos syndrome
– osteogenesis imperfecta
• Acquired disturbances
– lathyrism
-copper deficiency
– vitamin C deficiency
- Ehlers-Danlos syndrome group of inherited disorders characterized by hyperextensibility of skin, and abnormal tissue fragility.
- Alport syndrome is due to a defect in the formation of type lV collagen fibers found in the basement membrane of renal glomeruli. The patients exhibit hematuria and renal diseases.
- Osteogenesis imperfecta-characterized by abnormal fragility of bones due to decreased formation of collagen.
- Epidermolysis bullosa due to alteration in the structure of type Vll collagen. Presented with skin breaks and blisters formation even for a minor trauma.
- Scurvy-improper collagen synthesis due to deficiency of ascorbic acid. Characterized by bleeding gums, poor wound healing, and subcutaneous hemorrhage.
Elastin
-Important protein of connective tissue mainly responsible for the extensibility and elasticity of tissue.
-found in large quantities in Iungs, arterial blood vessels, elastic ligaments, etc
-Elastin is synthesized as tropoelastin which undergoes post-translational modifications
-the structure of elastin is simple compared to collagen without any repeat helix of (GIy-X-Y)n.
Elastic fibers
•are composed of a protein called elastin and a glycoprotein framework called fibrilin.
• They are very stretchy and branch and join to form a network.
• Can stretch up to 150 times its relaxed size
• They provide strength to tissues but allow the tissue to be flexible and stretchy. They are found in skin, blood vessels, and lungs
Occurrence
• in arteries, particularly in the aorta
• in the skin, tendons and loose connective tissue (relatively low content)
• in the lungs Synthesis takes place in early development or after tissue damage Half-time is approximately 70 years (lower content in elderly people).
Elastin Properties
Extensibility and contractility
▫ resembles the rubber
▫ After extension elastin can return to its original size and original form
▫ Tensile strength is lower than in collagen
▫ hydrophobic, practically insoluble in aqueous solutions
Primary structure
Occurrence of amino acids
▫ 1/3 glycine
▫ High content of nonpolar AA (Ala, Val, Leu, Ileu)
▫ low hydroxyproline
▫ No hydroxylysine − elastin is not glycosylated Sequence of amino acids
▫ Typical triplet as in collagen is not present Alternation of short hydrophobic and hydrophilic sections. Hydrophilic sections, which represent a minority part, are rich in lysine, which takes part in forming cross-links. Secondary and tertiary structure of elastin
Secondary structure
▫ elastin does not form a regular secondary structure
▫ elastin has a character of random coil conformation enabling extension and contraction of Tertiary structure
▫ A stable secondary structure is not expressed
Elastin synthesis
– Synthesis of polypeptide chain Hydroxylation of proline residues– Secretion of tropoelastin –Tropoelastin (globular structure, Mr = 70 000 )– Formation of cross-links Three-dimensional netting
Cross-links in elastin
-Cross-links
▫ There is a large number of covalent cross-links in elastin
▫ Some are similar to collagen
▫ The key step is oxidative deamination of some lysine residues by copper-containing lysyl oxidase (the same enzyme as in the formation of cross-links in collagen)
▫ cross-links may be formed within one polypeptide chain or between 2 – 4 chains
• Desmosine
▫ cross-link completely specific for elastin
▫ arises from 4 side chains of LYSINE (3 oxidized and 1 nonoxidized)
▫ determines the high elasticity of elastin
– Linking of polypeptide chains of elastin by cross-links constitutes a three-dimensional netting explaining the „rubber-like“ properties of elastin.
Abnormalities associated
-Williams syndrome is a genetic disease due to impairment in elastin synthesis. The connective tissue and central nervous system are affected.
-Decreased synthesis of elastin –in aging and pulmonary emphysema.
Fibrillin
-Fibrillin is a structural component of myofibrils found in various tissues
Associated syndrome
Marfan syndrome is a genetic disorder due to a mutation in the gene for fibrillin. it is characterized by hyperextensibility of joints and skeletal. system. Consequently, the patients of Marfan syndrome are tall and have long digits. These patients may also have cardiovascular complications.
Fibronectin
– Fibronectin, a glycoprotein, is closely involved in the interaction of cells with extracellular matrix. lt actively participates in cell adhesion and cell migration. In general, tumor cells are deficient in fibronectin which results in the lack of adhesion among the tumor cells that may often lead to metastasis
Laminin
The basal lamina of the glomerular membrane (of renal cells) contains laminin. Laminin is one of the first extracellular proteins synthesized during embryogenesis. it is actively involved in neuronal growth and nerve regeneration. ln the patients of Alzheimer’s disease, high concentrations of laminin are found.
Reticular fibers
• Made up of collagen but are thinner as compared to collagen fibers and are arranged in branching network (not in parallel rows as are collagen fibers).
• They form a spongelike framework, stroma, for such organs as the spleen and lymph nodes
• Also present in blood vessels, nervous tissue, muscles, and adipose tissue where it provides support.
References
- Ferrier D. R. & Harvey R. A. (2014). Lippincott’s illustrated reviews: biochemistry (6th ed.). Wolters Kluwer Health : Lippincott Williams & Wilkins.
- https://www.etsu.edu/uschool/faculty/tadlockd/documents/ant_connective_student_notes.pdf.
- http://www.uop.edu.pk/ocontents/connective%20tissues-converted.pdf
- https://ulbld.lf1.cuni.cz/file/2385/biochemistry-of-connective-tissue-dentistrykopptx.pdf
