Cope rearrangement of 1,5-dienes is one of the most fundamental C-C bonds-formation reactions available to synthetic chemists today. It is exceptional in the formation of highly stereospecific and predictable C-C σ -bonds between non functionalized carbon centers. Elizabeth Hardy and Arthur C Cope developed this rearrangement reaction and this was named after Arthur C Cope.
Interesting Science Videos
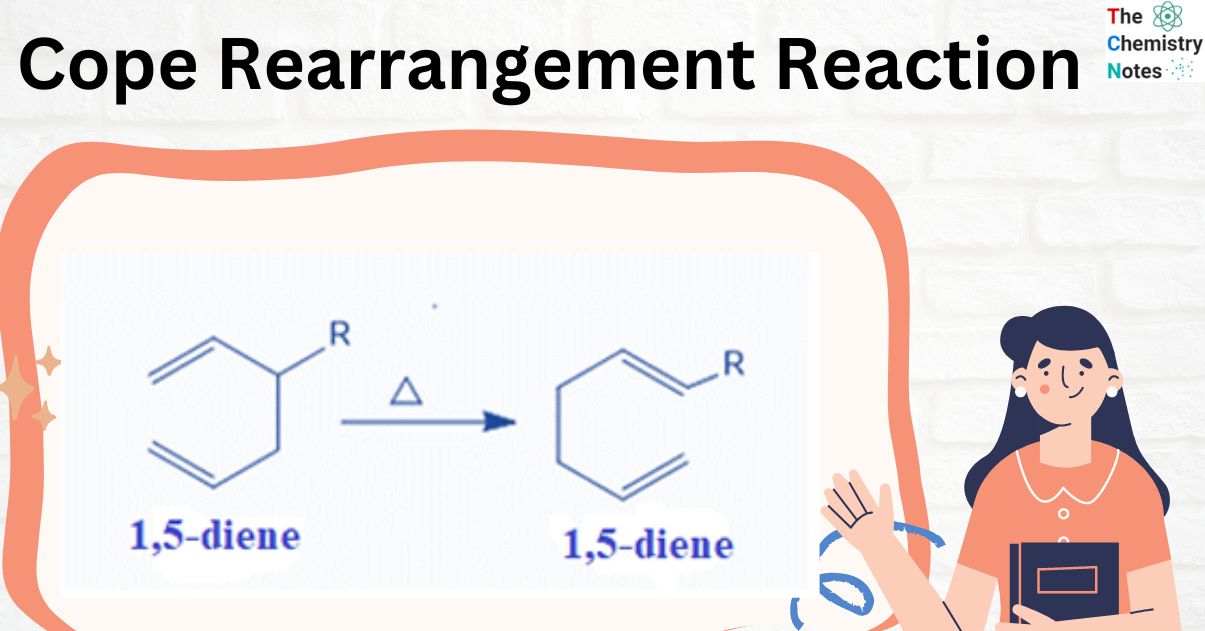
Cope Rearrangement Reaction – Definition
The Cope rearrangement is an organic reaction in which a 1,5-diene structural isomer is converted to another 1,5-diene structural isomer under thermal conditions. This reaction belongs to a class of reactions termed “sigmatropic rearrangements.”
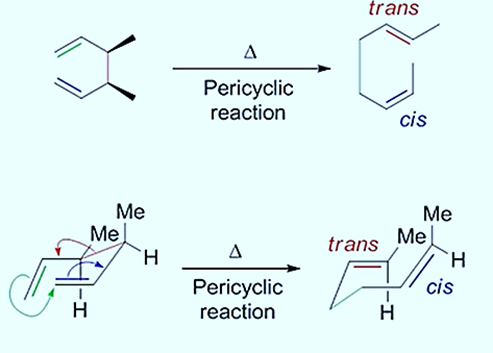
The stereochemistry of this reaction can be predicted when substituents are present in the initial diene starting material by modeling the molecule in a chair-like conformation. The equatorial position of the substituents reduces steric interactions and produces the major product. The overall stability of the starting material and the finished product determines the equilibrium position of the Cope rearrangement, which is an equilibrium process.
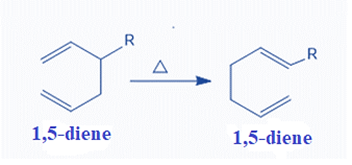
Mechanism of Cope Rearrangement Reaction
The Cope Rearrangement mechanism is depicted below. A π bond attacks the carbon six carbons away in this instance, creating a new σ bond. The second π bond is displaced as a result, and as the π bond migrates, a σ bond is cleaved and changes into a π bond.
Though the diradical is typically not a true intermediate, it can be helpful to think of it as transitioning through a state that is energetically and structurally equivalent to a diradical (potential energy minimum).
Stereochemistry
The Cope Rearrangement proceeds through a chair transition state, which can have stereochemical ramifications
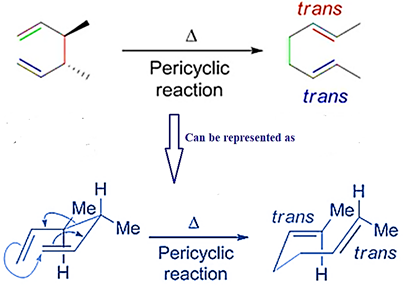
Thus, different stereochemistry of reactant will lead to different products.
Oxy Cope Rearrangement
The [3,3]-sigmatropic variation of the Cope rearrangement in which 1,5-dien-3-ols are changed into unsaturated carbonyl compounds by a typical mechanism is known as Oxy cope rearrangement.
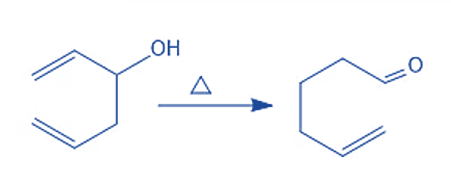
It entails restructuring the skeleton of a few unsaturated alcohols. The reaction is extremely versatile because it easily and predictably reorganizes a wide range of precursors, making it a very valuable synthetic as well. The spontaneous keto-enol tautomerization that produces a carbonyl is the driving force.
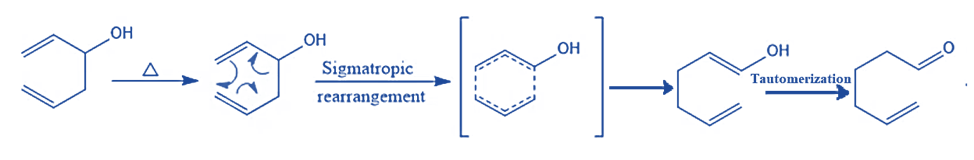
Mechanism of Oxy Cope Rearrangement
Application of Cope Rearrangement
Cope rearrangement is one of the most important organic chemistry rearrangement reactions. For more than 30 years, it has piqued the interest of many, particularly in organic synthesis. The most common method is to prepare macrocyclic compounds. For example, in the chemical field, how to effectively synthesize organic compounds with seven-membered structures is an important subject in the organic synthesis system. It has piqued the interest of chemists due to its mild reaction conditions, ease of raw material availability, and high yield, as well as its relatively high regioselectivity and stereo-independence.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- Stepwise Mechanisms in the Oxy-Cope Rearrangement Jerome A. Berson and Maitland Jones pp 5017 – 5018; J. Am. Chem. Soc. 1964;
- https://en.wikipedia.org/wiki/Cope_rearrangement
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- Application of Cope rearrangement in synthesis: Synthetic Communications: DOI: 10.1080/00397911.2019.1657460
