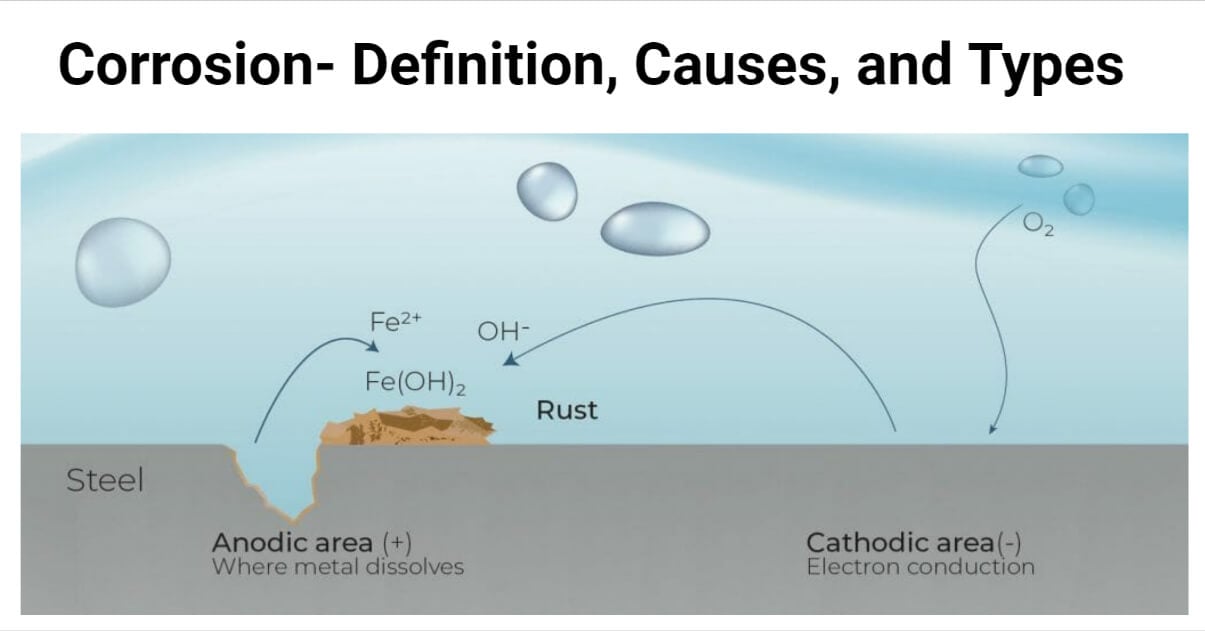
Corrosion is an undesirable phenomenon that destroys the luster and beauty of objects and shortens the life of materials. Thus, corrosion is defined as the destructive attack on the surface of metallic materials by chemical or electrochemical reactions with the environment leading to their degradation.
IUPAC defines corrosion as “An irreversible interfacial interaction of materials (metallic, ceramic or polymer) with their environment which results in the consumption of the materials”.
Interesting Science Videos
Causes of corrosion
Corrosion is the degradation of metals or alloys caused by environmental interaction. The presence of moisture, electrolytes, scratches, and cracks on the metal surface, as well as the interfacial contact of dissimilar metals, can cause corrosion. Different factors can accelerate the process of corrosion. They are as follows:
- Low pH
- High temperature
- Presence of electrolyte
- The surface of metal itself
- Moisture
Importance of corrosion study
Corrosion can cause a heavy loss in economy, materials, and failures of equipment and infrastructures. The main reason for the importance of corrosion study are as follows:
Economy factor
The loss of materials leads to economic loss. According to studies, developed countries lose 3-4.5 percent of their GDP due to corrosion. As a result, it is critical to study corrosion to reduce the economic loss caused by it.
Material designing
It is critical in engineering to design materials so that corrosion and material loss can be minimized.
Preservation of materials
Metals such as iron, aluminum, copper, and others can be mined and preserved with the proper knowledge about corrosion.
Types of corrosion
The types of corrosion can be classified based on the corroded substance, corrosive environment, and mechanism.
Types of corrosion based on the corroded surface
Uniform or general corrosion
It is a common form of corrosion and is characterized by chemical or electrochemical reactions which proceed uniformly over the entire exposed surface. E.g., rusting of iron or iron-based alloys. It is easy to measure and design against this type of corrosion damage. It is relatively easy to control by using protective coatings, inhibitors, and cathodic protection.
Pitting corrosion
Pitting corrosion is a localized form of corrosion by which cavities or holes, or pits are produced in the materials. Pitting is considered more dangerous because corrosion products may cover small pits or holes, often resulting in sudden and unexpected failure. Pitting corrosion is most aggressive in solutions containing chloride, bromide, or hypochlorite ions.
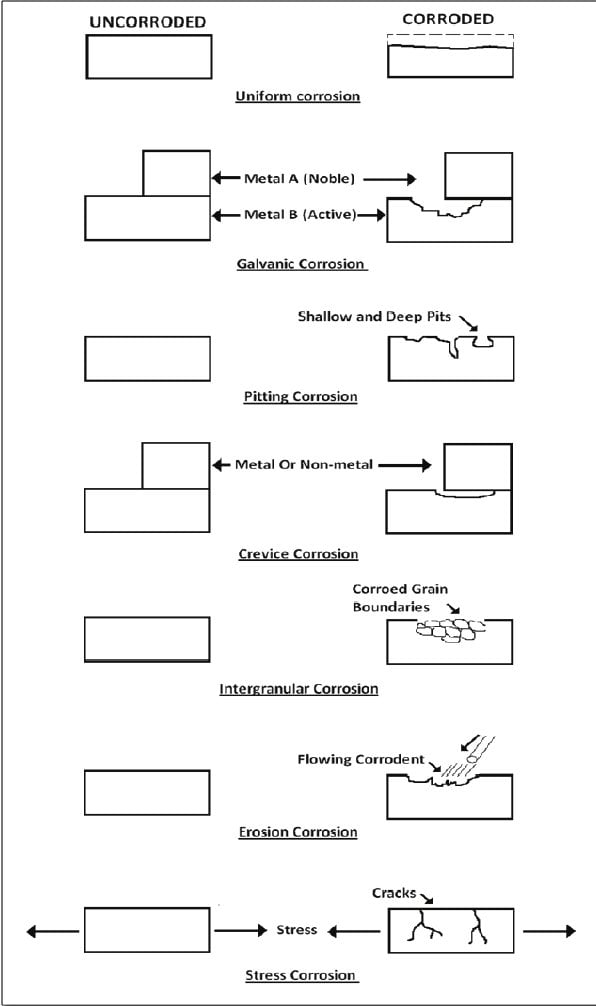
Galvanic corrosion
Galvanic corrosion occurs when two dissimilar metallic materials are brought into contact in the presence of the electrolytes. It occurs when two metals with different potentials are joined. The driving force for corrosion is a potential difference between two different metals. The corrosion is caused due to the formation of the galvanic cell, so it is called galvanic corrosion.
Environmental cracking
This occurs due to different environmental conditions which negatively affect different metals. Fatigue corrosion, stress corrosion cracking, and hydrogen embrittlement are the different types of environmental corrosion.
Flow assisted corrosion
Flow-assisted corrosion occurs When an oxide layer on a metal surface is dissolved or removed by wind or water, and the underlying metal is exposed to further corrosion and deterioration.
Selective leaching or de-alloying
It involves the selective removal of one element from an alloy by the corrosion process. E.g., Dezincification, graphitization.
Intergranular corrosion
Intergranular corrosion is the accelerated corrosion of metal along its grain boundaries while the bulk of the metal surface remains unaffected. Such corrosion is typically associated with the chemical segregation effect of a specific phase precipitated on grain boundaries, which can produce a zone of reduced corrosion resistance in the immediate vicinity. attack.
Crevice corrosion
It is a localized form of corrosion and usually results from a stagnant microenvironment in which there is a difference in the concentration of ions between two areas of metals. Crevice corrosion occurs in shielded areas under washers, bolt heads, and gaskets, where oxygen is restricted.
Erosion corrosion
It is an increase in the rate of deterioration or attack on metal caused by the relative movement of the corrosive fluid and the metal surface. In this case, the metal is removed from the surface as a dissolved ion or solid corrosion product.
Stress corrosion cracking
Stress corrosion cracking involves the formation of fine cracks on specific areas of the metal surface due to the simultaneous presence of tensile stresses in a corrosive environment.
Types of corrosion based on the corrosive environment
Based on the corrosive environment, there are four different types of corrosion. They are as follows:
Atmospheric corrosion
Atmospheric corrosion is the corrosion of materials exposed to air and its pollutants. It depends on the electrolytes, which may be rain, humidity, dew, or melting snow.
Aqueous corrosion
The corrosion of material immersed in water is called aqueous corrosion. The impurities present in the water are the main promoting factors of aqueous corrosion.
Soil corrosion
Corrosion of buried steel or iron pipe doe the supply of water, gas, and petrochemicals in the soil is called soil corrosion. Soil is of major importance for soil corrosion. The most corrosive soils have high moisture content, high electrical conductivity, high acidity, and high dissolved salts.
Types of corrosion based on the mechanism
There are two types of corrosion which are categorized based on the mechanism.
Dry corrosion
It is a chemical process caused by the direct interaction of metals with atmospheric gases like oxygen, H2S, SO2, and halogen. When these gases come into contact with a metal surface, it gradually begins to decolorize, leading to degradation. The formation of metal oxides, halides, or sulfide firms causes decolorization. It is primarily due to the presence of oxygen.
Wet corrosion
It has an electrochemical nature and is more common in wet than in dry conditions. It is found in aqueous environments. The metal surface that comes into contact with moisture oxidizes into metal ions, so the metal surface that comes into contact with air serves as a cathode. When exposed to moist air, iron corrodes to form reddish bone rust. It’s a typical example of wet corrosion.
References
- https://byjus.com/jee/corrosion/
- https://www.vedantu.com/iit-jee/corrosion
- https://www.aakash.ac.in/important-concepts/chemistry/galvanic-corrosion
- https://www.forging.org/forging/design/323-corrosion-environment.html
- https://quarkscience.com/corrosion
- https://www.thoughtco.com/types-of-corrosion-2340005
- https://www.corrosionpedia.com/the-8-most-common-forms-of-metal-corrosion/2/1680
- https://eoncoat.com/corrosion-that-you-can-see/
- https://www.gibsonstainless.com/types-of-corrosion.html
