Decomposition reactions are a type of chemical reaction in which a single compound breaks down into two or more simpler substances, which can be elements or compounds. They are the reverse of combination reactions, which involve the combination of two or more substances to form a single compound.
Interesting Science Videos
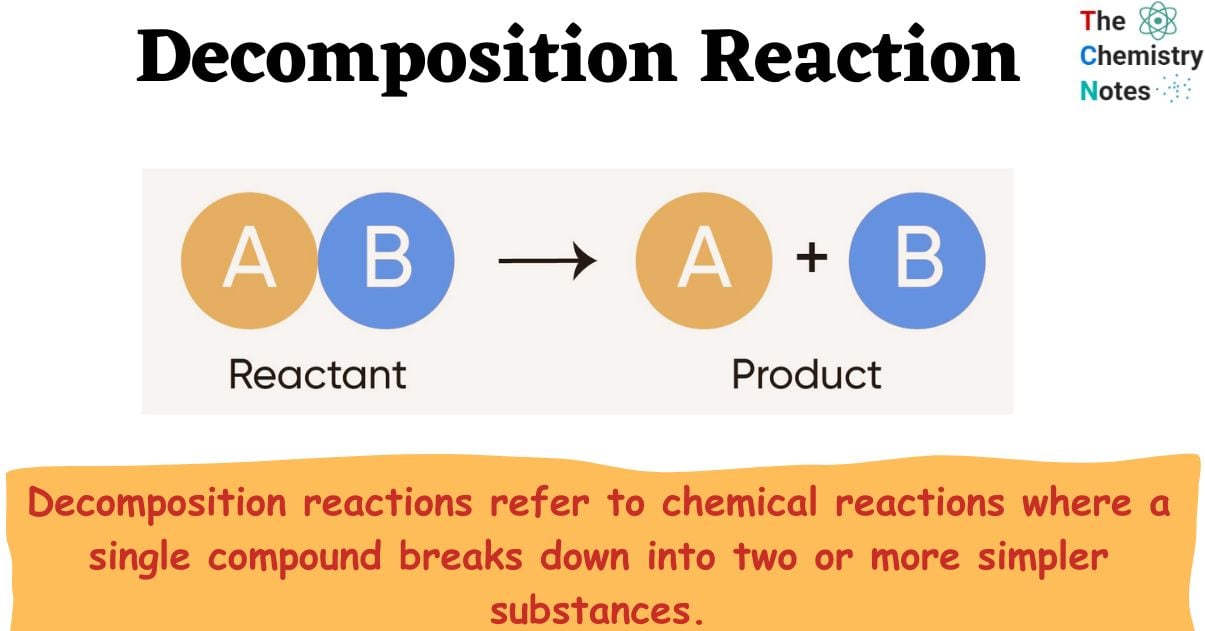
What is Decomposition Reaction?
Decomposition reactions refer to chemical reactions where a single compound breaks down into two or more simpler substances.
General reaction of Decomposition Reaction:
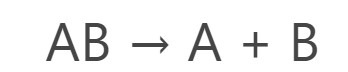
Specific chemical reaction of decomposition reaction:
2 H2O2 → 2 H2O + O2
Na2CO3 → Na2O + CO2
Since the decomposition process involves heat, it is often referred to as a thermal decomposition reaction. Heat, light, or electricity can all be used to provide the energy needed to break down the product. The electrolysis process is a great example of a decomposition reaction.
There are two types of decomposition or we can say changes, which are given below.
Physical Decomposition / Changes:
Physical changes are usually reversible, but sometimes they can also be irreversible, and only the physical properties are changed. In a physical change, a substance changes its properties. Some common examples of physical changes are melting an ice cube, boiling water, breaking a glass, freezing water, etc.
Chemical Decomposition / Changes:
Chemical changes are irreversible changes in which new products are formed. The product is different from the reactants and has entirely different properties. Chemical changes that occur are also considered chemical reactions. A way of describing a chemical reaction or change is through chemical equations. Chemical equations are a symbolic representation of a chemical reaction.
Types of Decomposition Reaction
There are three main types of decomposition reactions:
- Thermal decomposition,
- Electrolytic decomposition, and
- Photolytic decomposition.
Thermal Decomposition / Thermolysis
A thermal decomposition reaction is a decomposition reaction that uses thermal energy as its catalyst.
It is a type of decomposition in which a single reactant is broken down into more than one product with the help of heat. This is an endothermic reaction, as heat is used to break the bonds of the reactants.
Examples of a thermal decomposition reaction is provided below.
Decomposition of calcium carbonate: When calcium carbonate (CaCO3) is heated, it decomposes into calcium oxide (CaO) and carbon dioxide (CO2). The decomposition process produces quick-lime, which is used in several industries.
CaCO3 → CaO + CO2
Decomposition of potassium chlorate: When heated strongly, potassium chlorate decomposes into potassium chloride and oxygen. This reaction is used for the preparation of oxygen.
2 KClO3 (s) → 2 KCl (s) + 3 O2 (g)
Decomposition of ferric hydroxide: Ferric hydroxide undergoes decomposition in heat, giving ferric oxide and water molecules.
2 Fe(OH)3 → Fe2O3 + 3 H2O
Decomposition of hydrated oxalic acid: when heated, hydrated oxalic acid (H2C2O4.2H2O) decomposes into oxalic acid and water.
H2C2O4.2H2O → H2C2O4 + 2 H2O
Electrolytic decomposition / Electrolysis
Electrolytic decomposition may result when an electric current is passed through an aqueous solution of a compound.
The activation energy required for the breakdown process in this sort of decomposition reaction is provided in the form of electrical energy. An electric current is transferred via the electrolytic decomposition reaction in an aqueous solution of a chemical compound.
Examples of electrolytic decomposition reaction:
Electrolysis of Water: Electrolysis of water is the decomposition of water into hydrogen and oxygen due to the passage of an electric current.
2 H2O (l) → 2 H2 (g) + O2 (g)
![Electrolysis of water [Thermal decomposition reaction]](https://scienceinfo.com/wp-content/uploads/2023/05/image-164.png)
Decomposition of sodium chloride: On passing electricity through molten sodium chloride, it decomposes into sodium and chloride.
2 NaCl → 2 Na + Cl2
Photolytic decomposition / Photolysis
A photolysis reaction is a decomposition reaction in which the reactants absorb energy from photons to break down into their constituent parts.
It is a decomposition reaction in which light or photons break the reactants into several products. Light energy is used to break the bonds in the reactant. This reaction is also known as photolysis.
Examples of photolytic decomposition reaction:
Breakdown of ozone: The chemical equation below illustrates the breakdown of ozone into dioxygen and oxygen radicals as an illustration of a photolysis reaction.
O3 + uν → O2 + O
Decomposition of silver chloride: Place a small quantity of silver chloride (AgCl) in a watch glass under sunlight for some time. The crystals slowly acquire a gray color. On analysis, it is found that the sunlight has caused the decomposition of silver chloride into silver and chlorine.
2 AgCl (s) → 2 Ag (s) + Cl2 (g)
Silver bromide also decomposes in the same way when exposed to light. It changes its color from light yellow to grey
2 AgBr (s) → 2 Ag (s) + Br2 (g)
Decomposition of hydrogen peroxide: In the presence of light, hydrogen peroxide decomposes into water and oxygen.
2 H2O2 (l) → 2 H2O (l) + O2 (g)
Balancing Decomposition Reactions
One approach to balancing a decomposition reaction involves ensuring that the number of atoms of each element is equal on both sides of the equation. This can be achieved by adjusting the coefficients of the reactants and products as needed. It is important to note that the law of conservation of mass must be upheld throughout the balancing process.
Let us consider an example of decomposition reaction.
Decomposition of potassium chlorate (KClO3) into potassium chloride (KCl) and oxygen (O2).
KClO3 (s) → KCl (s) + O2 (g)
This is an unbalanced equation. To balance the equation, following steps are followed.
- Step 1: it is necessary to look at the quantity of oxygen (O) atoms present on the right-hand side of the equation and compare it to the quantity on the left-hand side. It is observed that there exists a disparity in the number of oxygen atoms, with two atoms on the right-hand side and three atoms on the left-hand side. To clarify, the right operand is multiplied by a factor of 3, while the left operand is multiplied by a factor of 2. It should be noted that the entire compound KClO3 will be multiplied by a factor of 2 and the oxygen will be balanced.
2 KClO3 (s) → KCl (s) + 3 O2 (g)
- Step 2: It is evident that there is an imbalance between the quantities of potassium (K) and chlorine (Cl). The equation exhibits a discrepancy in the number of atoms of K and Cl on either side. Specifically, the left-hand side contains two atoms of each element, whereas the right-hand side contains only one atom of each. The final equation is obtained by multiplying the compound KCl by a factor of 2.
2 KClO3 (s) → 2 KCl (s) + 3 O2 (g)
- The chemical equation presented is a representation of the reaction between two molecules of potassium chlorate (KClO3) in the solid state, which results in the formation of two molecules of potassium chloride (KCl) also in the solid state and three molecules of oxygen (O2) in a gaseous state.
Rules for Decomposition Reactions
The following guidelines can be used to estimate the results of a chemical equation for decomposition:
- Upon heating, carbonates undergo decomposition to yield an oxide and carbon dioxide.
CaCO3 ⟶ CO2+CaO
- Upon heating, chlorates undergo decomposition to yield chloride and oxygen gas.
2KClO3 ⟶ 3O2 + 2KCl
- Upon heating, hydroxides undergo decomposition to yield oxide and water.
2Fe(OH)3 ⟶ 3H2O+Fe2O3
- Upon heating, acids undergo decomposition into molecular oxide and water. (This applies exclusively to acids that possess oxygen.)
H2SO4 ⟶ H2O + SO3
- Upon heating, oxides undergo decomposition to yield oxygen and a corresponding element.
2HgO ⟶ O2 + 2Hg
- A compound disintegrates into its individual components when the elements are separated using electricity.
MgCl2 ⟶ Mg + Cl2
Uses Of Decomposition Reaction
The decomposition reaction is widely used in different fields. Some of the uses are discussed below:
Used In The Production Of Oxygen
The decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2) is an example of a decomposition reaction. This reaction is widely used to produce oxygen for medical and industrial purposes.
Used For The Production Of Chemicals
Decomposition reactions are employed in the synthesis of different compounds. Calcium carbonate (CaCO3), for example, decomposes to create calcium oxide (CaO) and carbon dioxide (CO2). Calcium oxide is used in the manufacture of cement as well as other compounds.
Used For The Preservation Of Food
Decomposition reactions are used in the preservation of food. Sodium nitrite (NaNO2) decomposes to produce nitric oxide (NO) and nitrogen dioxide (NO2) which act as preservatives in meat products.
Used For The Production Of Fertilizers
Decomposition reactions are used in the production of fertilizers. Ammonium nitrate (NH4NO3) decomposes to produce nitrogen gas (N2) and water (H2O). The nitrogen gas is used by plants for growth and the water helps in the process of photosynthesis.
Used As a Pollutants Remover
Decomposition reactions are used to remove pollutants from the environment. For example, ozone (O3) decomposes to produce oxygen (O2) and atomic oxygen (O). Atomic oxygen reacts with pollutants such as nitrogen oxides (NOx) and sulfur dioxide (SO2) to form harmless products.
Frequently Asked Questions (FAQ)
What are some real-life examples of decomposition reactions?
1. Car airbags inflate as a result of sodium azide reaction with breakdown. The sodium azide inside the airbag reacts with a modest quantity of heat when a sensor on the car senses a collision. The chemical then disintegrates, or decomposes, into sodium and nitrogen gas as a result of this heat energy. The airbag is inflated by this gas, which also shields the occupants from the collision’s force.
2. In order to break down lipids, carbs, and proteins, the human body frequently undergoes decomposition events.
3. The silver bromide coating on photographic film breaks down into silver and bromine when it is exposed to light. This is how the film develops into a visible image.
Why are Decomposition Reactions referred to as the opposite of Combination Reactions?
The decomposition reaction is the combination reaction’s exact opposite. A material is created through a chemical reaction known as a combination reaction, whereas a decomposition reaction results in the formation of new substances.
What is the difference between Combination and Decomposition Reactions?
Combination reactions combine the reactants to create the product, which is the primary distinction between them and decomposition reactions. In contrast, one reactant might split into two or more products during a breakdown process.
Are all processes involving decomposition endothermic?
Energy in the form of heat, light, or electricity is needed for the majority of decomposition reactions. Breaking of the bonds in the reacting substance due to energy absorption results in the product’s decomposition. However, the process of decomposition doesn’t always involve the transfer of heat. During decomposition, both exothermic and endothermic events can occur. However, the decomposition reaction endothermic is more common than the exothermic.
Which is a characteristic of a decomposition reaction?
The fact that there is just one reactant in the equation distinguishes a decomposition reaction from other types of reactions.
Video on Decomposition of Hydrogen Peroxide
References
- Atkins, Peter W.; Julio de Paula (2006). Physical Chemistry (4th ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-31546-8.
- Wiberg, Egon, Wiberg, Nils and Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. ISBN 978-0-12-352651-9.
- https://testbook.com/chemistry/decomposition-reaction
- studysmarter.co.uk/explanations/chemistry/chemical-reactions/decomposition-reaction/
- https://collegedunia.com/exams/decomposition-reactions-electrolytic-photochemical-thermal-decomposition-chemistry-articleid-661
- https://byjus.com/chemistry/decomposition-reaction/
- https://www.inspiritvr.com/general-chemistry/chemical-reactions/decomposition-reaction-study-guide
- https://www.aakash.ac.in/important-concepts/chemistry/decomposition-reaction
- https://chemistrytalk.org/decomposition-reaction/

