Detergents, in their various forms of powder or liquid, have emerged as the preeminent cleansing agents for fabrics, enjoying widespread utilization within the realm of laundering. These cleaning agents effectively suspend, solubilize, dissolve, or separate dirt and soil from textiles, preventing them from sticking back to the outside of the fabric. Instead, these contaminants remain in a state of suspension within the aqueous medium.
Synthetic detergents, as they are often called, are made mostly of compounds that come from petrochemicals. They include wetting agents and emulsifiers, which are usually in the form of sulfate or sulfonate salts. Detergents exhibit remarkable efficacy in the eradication of dirt and soil particles from textiles composed of both synthetic and natural fibers.
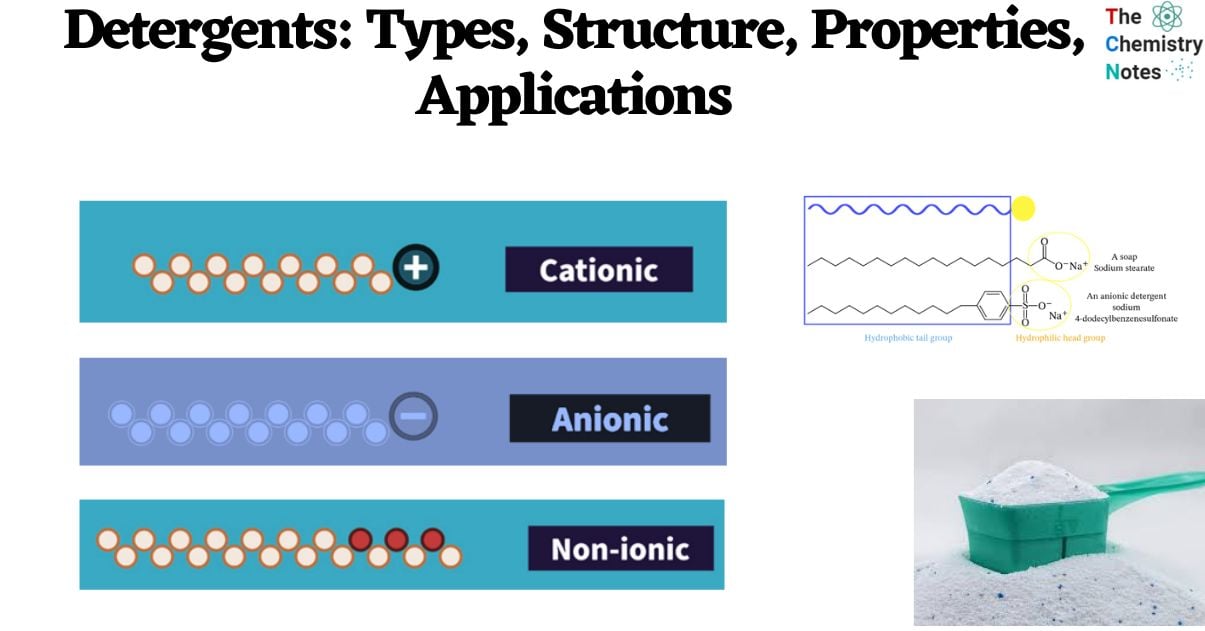
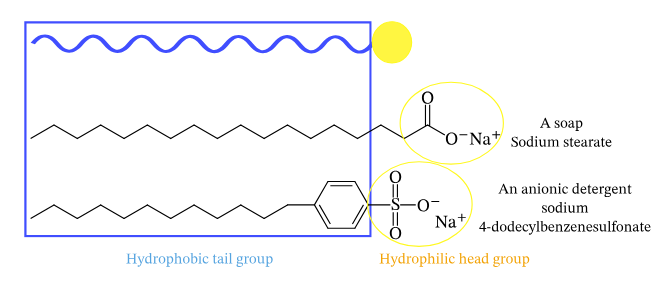
A detergent can be regarded as akin to soap, albeit possessing a more generalized structure denoted as R-SO4–, Na+, wherein R represents an elongated alkyl group. Similar to soap, detergents possess amphiphilic properties, meaning they possess both hydrophobic and hydrophilic regions. The majority of detergents in circulation are composed of alkylbenzene sulfonates. Detergents exhibit a higher degree of solubility in hard water compared to soap due to the differential behavior of their respective chemical components. In particular, the sulfonate in detergents is less likely to form strong bonds with calcium and other ions found in hard water, while the carboxylate in soap is more likely to do so.
Interesting Science Videos
History of Detergents
- The development of synthetic detergents took place in Germany during World War I. In response to the Allied Blockade of Germany in 1917, which resulted in a scarcity of soap-making components, an alkyl sulfate surfactant was developed.
- The term “detergent” originates from the Latin term “detergere” denoting the action of wiping away. Before the advent of detergent, washing soda or sodium carbonate was commonly employed for the purposes of dishwashing and laundering garments.
- In American history, it is recorded that the initial iteration of liquid dishwashing detergent emerged during the 1930s. Conversely, across the vast expanse of Europe, the genesis of a detergent specifically formulated for this noble purpose, known as Teepol, came to fruition in the year 1942.
- Laundry detergents were introduced concurrently, with options available in both solid and liquid states. Both dishwashing and laundry detergent consist of a wide array of additional compounds, commonly encompassing enzymes, bleach, fragrances, dyes, fillers, and, in the case of laundry detergent, optical brighteners.
- The inclusion of additives is imperative due to the inherent challenges faced by detergents in effectively eliminating dyes, pigments, resins, and denatured proteins. Reagent detergents utilized in the realm of biology typically manifest as refined iterations of surfactants.
Structure of Detergents
- Detergents encompass a class of chemicals characterized by an amphiphilic molecular structure, wherein each individual molecule possesses a hydrophilic (polar) head and an elongated hydrophobic (non-polar) tail.
- The hydrophobic portion of these molecules has the potential to display either linear or branched hydrocarbon structures, or it may feature a shape like that of a steroid.
- The hydrophilic component demonstrates a wider range of diversity, including both ionic and non-ionic properties, and exhibits a spectrum of structural complexity that ranges from simple to sophisticated arrangements.
- The characteristics of detergents are contingent upon the molecular configuration of the monomeric unit.
Properties of Detergents
- Detergents function as surfactants by reducing the surface tension of water. The inherent duality of their composition enables the amalgamation of hydrophobic substances, such as oil and grease, with aqueous solutions. Due to the hydrophobic nature of air, detergents serve as foaming agents to differing extents.
- Detergent molecules have the ability to aggregate and form micelles, thereby enhancing their solubility in water. The hydrophobic moiety of the detergent serves as the primary factor responsible for the formation of micelles. Its aggregation leads to the development of the hydrophobic core within the micellar structure.
- The micelle possesses the ability to efficiently remove grease, proteins, and other particulates that contribute to soiling. The critical micelle concentration (CMC) is defined as the concentration at which micelle formation initiates.
- Furthermore, the temperature at which the micelles undergo further aggregation, resulting in the separation of the solution into two distinct phases, is commonly referred to as the cloud point. At this juncture, the clarity of the solution diminishes and the detergency achieves its optimal level.
- Detergents demonstrate enhanced efficacy under alkaline pH conditions. The composition of the head group can impact the foaming characteristics of surfactants. Anionic surfactants tend to exhibit high foaming properties, whereas nonionic surfactants typically have non-foaming or low-foaming attributes.
Types of Detergents
Detergents can be categorized into three primary groups based on the electrical charge exhibited by their surfactants.
Anionic Detergents
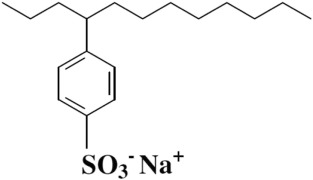
- Alkyl benzene sulfonates (ABS) are frequently found as anionic detergents. The alkylbenzene component of these anions exhibits lipophilic properties, while the sulfonate group demonstrates hydrophilic characteristics.
- Approximately 6 billion kilograms of anionic detergents are manufactured annually for consumption in domestic markets.
- Two distinct varieties have gained popularity, namely those characterized by branched alkyl groups and those characterized by linear alkyl groups.

Non-Biodegradable (Branched Alkyl Benzene Sulfonates)
- Beginning in the early 1930s, branched alkylbenzene sulfonates (BAS) underwent a period of tremendous growth. These synthetic detergents were frequently referred to as syndets in early literature.
- In contrast to conventional soap products, BAS exhibits a remarkable capacity to withstand the adverse effects of hard water while also demonstrating enhanced foaming properties. Nevertheless, the intricate and extensively branched nature of the tail posed a considerable challenge in terms of its biodegradability.
- The formation of extensive stable foam in areas of wastewater discharge, including lakes, rivers, coastal areas (sea foams), sewage treatment facilities, and drinking water contamination, has been widely attributed to the presence of BAS.
- During the 1960s, linear alkylbenzene sulfonates (LAS) were introduced as a replacement for branched alkylbenzene sulfonates (BAS) in the majority of detergent products, resulting in the phasing out of BAS. In specific agrochemical and industrial contexts, the significance of rapid biodegradability may be diminished, yet it remains a crucial factor.
Bio-Degradable (Linear Alkyl Benzene Sulfonates)
- Linear alkylbenzene sulfonates (LAS) are derived through an industrial process involving the sulfonation of linear alkylbenzenes (LABs). The production of LABs can be accomplished through various methods.
- The designation “linear” pertains to the initial alkenes as opposed to the ultimate outcome. The aforementioned compound exhibits a significantly accelerated rate of biodegradation in comparison to BAS, thereby rendering it a more prudent selection in the long run.
- The substance in question undergoes rapid biodegradation when exposed to aerobic conditions, exhibiting a half-life of approximately 1 to 3 weeks.
- In an anaerobic environment, the decomposition of the substance proceeds at a slow rate or may cease altogether, leading to its substantial accumulation in sewage sludge.
- Nevertheless, it is widely acknowledged that this situation does not justify concern, as the substance will rapidly undergo degradation upon being reintroduced to an oxygen-rich environment.
Cationic Detergents
- Cationic detergents share similarities with anionic detergents due to the presence of a hydrophilic component. In contrast to anionic detergents that possess a sulfonate group, cationic surfactants are characterized by a polar end consisting of quaternary ammonium.
- Cationic detergents are synthesized via the chemical reaction between quaternary ammonium salts of amines and anions, such as acetate, chlorides, or bromides. The chains consist of cations that demonstrate solubility at the terminal position.
- These compounds are characterized by the presence of extended hydrocarbon chains with positively charged nitrogen atoms. The surface-active properties of these substances can be attributed to the presence of long-chain cations.
- One illustration of a cationic detergent is cetyltrimethylammonium bromide. This compound is commonly employed in hair conditioning products.

Non-Ionic Detergents
- Non-ionic detergents consist of hydrophilic head groups that lack an electrical charge. The head groups in these compounds may comprise polyoxyethylene moieties, detergents, or glycosidic groups, such as octyl glucoside and dodecyl maltoside.
- Non-ionic detergents are called non-denaturing agents because they can break up interactions between lipids and proteins but not between proteins themselves.
- Hence, these compounds find extensive application in the isolation of membrane proteins, allowing for the preservation of their biologically active conformation.
- In contrast to ionic detergents, salts barely affect the micellar size of non-ionic detergents.
- These detergents are devoid of any ionic constituents. These compounds can be classified as esters derived from alcohols with significant molecular mass.
- An exemplary detergent is synthesized through the chemical reaction between stearic acid and polyethylene glycol. Liquid dishwashing detergents exhibit non-ionic characteristics.

Zwitter Ionic Detergents
- Zwitterionic detergents possess characteristics that encompass both ionic and non-ionic attributes. Zwittergents, akin to non-ionic detergents, possess a lack of net charge, display no electrical conductivity or electrophoretic mobility, and do not manifest any binding affinity towards ion-exchange resins.
- Similar to ionic detergents, these substances are effective in disrupting protein-protein interactions.
- Zwittergents such as CHAPS, which are derived from steroids, have a lower tendency to degrade proteins compared to linear-chain zwittergents like dodecyl dimethyl diamine oxide.
- Zwitterionic detergents are characterized by a balanced charge, which is achieved through the presence of an equal number of positively charged (+1) and negatively charged (1-) chemical groups. One example of a widely used payment system is CHAPS (Clearing House Automated Payment System). CHAPS is an acronym that stands for: 3[(3cholamidopropyl)dimethylammonio]-1-propane sulfonate
Laundry Detergents
- Laundry detergent, commonly referred to as washing powder, is a specialized form of detergent designed specifically for the task of cleansing laundry items. Laundry detergent is manufactured in two primary forms: powdered and liquid formulations.
- At present, powdered and liquid detergents hold comparable market shares in the global laundry detergent industry in terms of their respective market values. Upon analyzing the sales volume, it becomes evident that powdered detergents are being sold at a rate that is twice as high as that of liquid detergents.
- Different types of detergent are used based on various factors such as the type of laundry, the level of dirtiness, the severity of stains, and the specific fabric requirements.
Components of Laundry Detergents
Laundry detergents often consist of various components, including builders (approximately 50% by weight), surfactants (15%), bleach (7%), enzymes (2%), soil anti-deposition agents, foam regulators, corrosion inhibitors, optical brighteners, dye transfer inhibitors, fragrances, dyes, fillers, and formulation aids.
Builders
- Builders, also known as chelating or sequestering agents, are substances that are used to soften water. Hard water is characterized by the presence of various minerals, including calcium, magnesium, and metallic cations such as iron, copper, and Manganese (Mn).
- When these positively charged ions react with negatively charged surfactant anions, insoluble compounds called metallic soaps or lime soaps are made. These compounds then precipitate onto fabrics and washing machines, posing a challenge when it comes to their removal.
- Builders employ various methods to eliminate hard water ions, such as precipitation, chelation, or ion exchange. Furthermore, they assist in the process of soil removal through dispersion.
- The initial substances utilized in the construction industry were Sodium Carbonate (Na2CO3), commonly known as washing soda, and sodium silicate, also referred to as water glass. Phosphates, specifically sodium phosphates, and polyphosphates, such as sodium hexametaphosphate, have been utilized since the 1930s. This trend has persisted with the subsequent introduction of phosphonates, including HEDP, ATMP, and EDTMP.
- In light of the recognized detrimental impact of these agents on the environment, there is a growing endeavor to transition towards phosphorus-free alternatives that are more environmentally friendly. Polycarboxylates like EDTA and NTA citrate like trisodium citrate, silicates like sodium silicate, gluconic acid, polyacrylic acid, and ion exchange agents like zeolites are all examples of these kinds of alternatives.
Surfactants
- Surfactants are chemical compounds that possess the capacity to decrease the surface tension or interfacial tension between various substances, including two liquids, a liquid and a gas, or a liquid and a solid. Surfactants possess the capacity to fulfill a range of functions, including emulsification, wetting, detergency, foaming, and dispersion.
- Surfactants play a crucial role as key constituents in laundry detergent, greatly enhancing its cleaning efficacy. This is achieved through the mechanism of absorbing and emulsifying soil particles into the water, simultaneously reducing the surface tension of the water to enhance its wetting characteristics.
- Laundry detergents primarily consist of anionic and non-ionic surfactants. Cationic surfactants typically exhibit incompatibility with anionic detergents, resulting in suboptimal cleaning performance. Consequently, their usage is limited to specific applications that require particular effects, such as fabric softening, reduction of static electricity, and antimicrobial properties.
- Zwitterionic surfactants are infrequently utilized in laundry detergents primarily due to cost considerations. The majority of detergents employ a blend of different surfactants in order to optimize their overall performance.
Enzymes
- In 1913, Otto Rohm made a noteworthy contribution to the field of laundry by introducing the application of enzymes. The preliminary step involved acquiring a pancreatic extract from animals that had undergone slaughter. Nevertheless, it was observed that this extract exhibited instability upon exposure to alkali and bleach.
- The widespread adoption of this technology occurred in the later half of the century, chiefly attributed to the advent of thermally resilient bacterial enzymes.
- Enzymes are essential for the degradation of stubborn stains composed of diverse substances, such as proteins (found in milk, cocoa, blood, egg yolk, and grass), fats (including chocolate, fats, and oils), starch (present in flour and potato stains), and cellulose (which can be found in damaged cotton fibrils, as well as vegetable and fruit stains).
- Various types of stains require the utilization of specific enzymes. Proteins stains can be effectively treated with proteases, such as savinases, whereas greasy stains necessitate the use of lipases. In contrast, α-amylases are effective in addressing carbohydrate stains, whereas cellulases are the preferred treatment for cellulose stains.
Bleaches
- Contrary to its name, contemporary laundry bleaches do not contain household bleach, also known as sodium hypochlorite. Laundry bleaches commonly consist of stable adducts of Hydrogen Peroxide, such as sodium perborate and sodium percarbonate. These substances remain inert in solid form but release hydrogen peroxide when they come into contact with water.
- The primary objectives of bleaches are to remove oxidizable organic stains, typically derived from plants. These stains may include chlorophyll, anthocyanin dyes, tannins, humic acids, and carotenoid pigments.
- Hydrogen peroxide exhibits limited bleaching activity at temperatures below 60°C, thereby necessitating the conventional use of hot washes. The advent of bleach activators during the 1970s and 1980s facilitated the efficacy of lower washing temperatures.
- These compounds, such as tetraacetylethylenediamine (TAED), undergo a chemical reaction with hydrogen peroxide, resulting in the formation of peracetic acid. Peracetic acid is known to possess enhanced bleaching properties, especially when utilized at lower temperatures.
Miscellaneous Additives
- Additional ingredients may be incorporated based on the anticipated usage conditions. These additives alter the foaming characteristics of the product by either stabilizing or mitigating foam formation.
- Additional ingredients can either enhance or diminish the viscosity of the solution or facilitate the solubilization of other ingredients. Corrosion inhibitors are utilized to mitigate the detrimental effects on washing equipment caused by corrosion.
- Dye transfer inhibitors are substances that effectively prevent the transfer of dyes from one article to other items, thereby preserving their original coloration. Antiredeposition agents, such as carboxymethyl cellulose, are employed to inhibit the reattachment of fine soil particles to the surface of the cleaned product.
- Several ingredients can impact the visual characteristics of the item to be cleaned or the detergent itself, either prior to or during its usage. The agents encompassed in this category consist of optical brighteners, fabric softeners, and colorants.
- Various fragrances are also included in contemporary detergents, as long as they are compatible with the other ingredients and do not have any impact on the color of the item being cleaned.
- Most perfumes are a mix of different chemicals, such as terpene alcohols like citronellol, geraniol, linalool, and nerol, as well as their esters like linalyl acetate. Additionally, aromatic aldehydes such as helional, hexyl cinnamaldehyde, and lilial, as well as synthetic musks like galaxolide, are frequently utilized in perfume.
Dish-Washing Liquids
- Typically, it is a formulation consisting of surfactants that produce abundant foam and have minimal skin irritation. Its primary purpose is to facilitate the manual cleaning of glassware, dishes, silverware, and culinary tools in a sink or basin.
- In addition to its primary function, dishwashing liquid also possesses a range of informal applications, including the generation of bubbles, laundering garments, and the cleansing of birds affected by oil.
- Hand dishwashing liquids are considered to be a highly significant category of household chemicals. Despite the widespread use of automatic dishwashers, a significant amount of dishwashing continues to be carried out manually. In the case of particularly challenging-to-clean items, it is generally recommended to wash them by hand instead of utilizing a dishwasher.
- Hand dishwashing detergents employ surfactants as the main agents responsible for the cleaning process. The diminished surface tension of dishwashing water, coupled with the enhanced solubility of contemporary surfactant blends, facilitates rapid water runoff from dishes placed in a dish rack.
Dish-Washing Detergents
- Dishwasher detergent is a specialized cleaning agent formulated specifically for the purpose of washing dishes in a dishwasher. Dishwasher detergent and dishwashing liquid designed for manual dishwashing are distinct from one another.
- When employing a dishwasher, it is crucial for the user to meticulously select a detergent that is appropriate for its designated purpose.
- Many dishwasher detergents are not suitable for use with silver, brass, cast iron, bronze, aluminum, pewter, and gold leaf materials.
- Dishwashing detergents designed for use in dishwashers are produced and sold in a variety of formats, including cartridges, gels, liquids, powders, and tablets. It is possible for dishwashing liquid to contain ingredients such as bleach, enzymes, or rinsing aids.
- Certain dishwashing detergents can be prepared at home by utilizing ingredients such as borax, essential oil, eucalyptus oil, and grated bar soap, among other potential components.
Components of Dish Washing Detergents
Various types of dishwashing detergent contain varying combinations of ingredients. Typical ingredients commonly found in various recipes include:
Alkaline Salts
- Alkaline salts are a crucial component found in conventional dishwasher detergent powders, especially in earlier and original formulations.
- Alkaline salts demonstrate the capacity to efficiently degrade and remove grease.
- Nevertheless, it is imperative to acknowledge that they display a significant degree of corrosiveness and might pose a potentially lethal risk if consumed.
- The salts employed may include metasilicates, alkali metal hydroxides, sodium carbonate, and other analogous compounds.
Phosphates
- It is recommended to use a method that involves sequestering calcium and magnesium ions to reduce the chance of limescale formation, which is often linked to the presence of hard water.
- The reason for this phenomenon is attributed to the well-established correlation between hard water and the formation of limescale deposits.
- Due to the environmental risks associated with certain compounds, certain substances have been subject to outright prohibition, while others are undergoing a gradual phase-out process.
Oxygen-Based Bleaching Agents
- Oxygen-based bleaching chemicals, commonly present in traditional powders and liquids, differ from chlorine-based bleaching agents.
- The process of disintegration and chemical treatment of organic accumulations
Enzymes
- Enzymes possess the ability to hydrolyze protein-based food residues, and they also have the potential to degrade oil, lipid, and fat deposits.
- The enzymes tested in this investigation demonstrate resemblances to those frequently exploited in washing applications.
Non-Ionic Surfactants
- Non-ionic surfactants are a type of surface-active agents that do not carry an electrical charge.
- This product effectively reduces the surface tension of water, facilitating the emulsification of oil, lipid, and fat food residues.
- Additionally, it helps prevent the formation of droplet spots during the drying process.
Miscellaneous Additives
- Anti-foaming agents are utilized to reduce the disruption caused by foam in the washing process.
- The existence of foam has the potential to affect the operational capabilities of the water-level sensors within the machine and could potentially lead to leakage through the door seals.
- The addition of additives is a potential strategy for reducing the rate of glaze and pattern degradation in glazed ceramics.
- Detergents typically contain a variety of ingredients, including perfumes, anti-caking agents (in the case of granular detergents), starches (in tablet-based detergents), gelling agents (in liquid and gel-based detergents), and sand (as the cost-effective option in powdered detergents).
Applications of Detergents
Household Uses
- One of the primary uses of detergents is in the field of household and commercial cleaning, which encompasses tasks such as dishwashing and laundry washing.
- These detergents are widely accessible in the form of powders or concentrated solutions. The compositions of these detergents typically consist of intricate combinations of various chemicals, in addition to surfactants.
- This is a result of the diverse requirements of different applications and the intense competition within the consumer market.
Used As Fuel Additives
- Both carburetors and fuel injector components of internal combustion engines benefit from the addition of detergents in fuels, as they effectively prevent fouling.
- The concentrations are estimated to be around 300 parts per million (ppm).
- Long-chain amines and amides, such as polyisobutene amine and polyisobutene amide/succinimide, are frequently employed detergents in a wide range of applications.
Used As Biological Reagent
- The process of isolating and purifying integral membrane proteins from biological cells involves the use of reagent-grade detergents.
- The solubilization of cell membrane bilayers requires the utilization of a detergent that has the capability to penetrate the inner membrane monolayer.
- The continuous improvement in the quality and complexity of detergents has greatly assisted the exploration and understanding of the composition and biophysical properties of prominent membrane proteins.
- Proteins play a crucial role in membrane disruption through their interaction with lipopolysaccharide, particularly through the involvement of ion channels.
- In addition, the application of detergents has enabled the investigation of transporters, signaling receptors, and photosystem II, all of which play vital roles in membrane functionality.
Video on Environmental Impacts of Detergents
References
- https://www.embibe.com/exams/detergents/
- https://www.thoughtco.com/definition-of-detergent-in-chemistry-604428
- https://www.cleancult.com/blog/different-types-of-detergent
- https://www.toppr.com/ask/en-np/content/concept/detergents-203561/
- https://www.nagwa.com/en/explainers/469184539393/
- http://www.chemistryexplained.com/Co-Di/Detergents.html

