Diamond is the purest crystalline carbon allotrope. It has a number of carbons that are bonded together tetrahedrally. Each tetrahedral unit is made up of carbon that is bonded to four carbon atoms, which are then attached to other carbons. This results in a carbon allotrope with a three-dimensional arrangement of C-atoms. Diamonds are formed by great heat and pressure deep beneath the Earth’s surface, which contributes to the mineral’s glitter and shine.
Interesting Science Videos
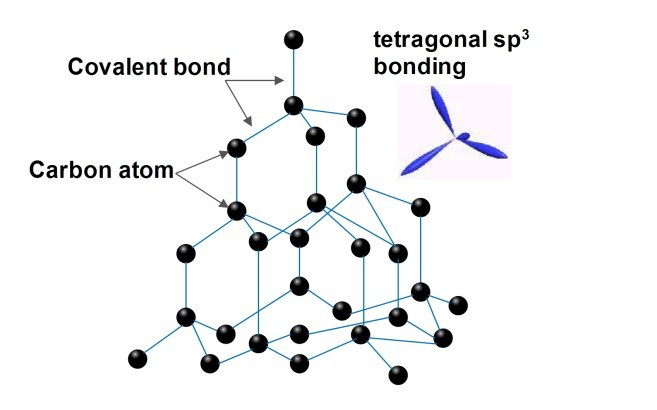
Structure of Diamond
Diamond is a carbon allotrope that is solid. A solid is a state of matter in which the molecules are closely packed together and have a distinct structure. Diamond is one of the hardest forms of carbon and is primarily employed in the creation of magnificent gemstones.
- The carbon atoms in diamond are said to generate strong chemical bonds with the other four carbon atoms, resulting in a perfect tetrahedron structure that extends throughout the crystal.
- Each diamond carbon atom has four covalent bonds which are quite strong. High temperatures are required to give the energy required to break them down. As a result, diamonds have a very high melting point.
- Carbon atoms are sp3 hybridised, and the lengths of carbon-carbon atom bonds are equivalent. As a result, Diamond develops a three-dimensional network of strong covalent bonds
[Image source: http://dx.doi.org/10.13140/RG.2.1.2971.0242
Crystal structure of Diamond
- A diamond’s crystal structure is a face-centered cubic or FCC lattice. In typical tetrahedrons (triangular prisms), each carbon atom connects four other carbon atoms.
- Diamond crystals can form into a variety of shapes known as ‘crystal habits’ due to its cubic form and highly symmetrical atom arrangement. The eight-sided octahedron or diamond form is the most frequent crystal habit.
- Diamond crystals can also take the shapes of cubes, dodecahedra, and combinations of these.
- Real diamond crystals feature raised or indented triangular growths termed ‘trigons’ on their faces rather than totally smooth faces.
- Diamonds have flawless cleavage in four directions, which means they will separate perfectly along these lines rather than breaking in a jagged fashion. The cleavage lines appear because the diamond crystal has fewer chemical bonds in the plane of its octahedral face than in other orientations.

[Image Source: 123dartist/stock.adobe.com
Physical Properties of Diamond
Diamond is a naturally occurring mineral made of carbon atoms organized in a crystalline lattice structure. As a result, it has a number of distinct physical characteristics.
- Diamond is the hardest known natural substance, with a Mohs hardness grade of 10. This means that it cannot be scratched or harmed by anything other than another diamond.
- Diamond has a very high melting point of roughly 3,500 degrees Celsius, making it exceptionally resistant to heat and thermal shock.
- Diamond has a high refractive index, which means it bends and slows light down more than most other materials. This attribute is responsible for diamond’s distinctive glitter and brightness, which is highly valued in jewelry.
- Diamond has a high dispersion, which means it breaks up white light into its constituent hues, producing a rainbow-like appearance known as fire.
- Diamond is transparent to visible light, which means it lets light travel through without dispersing or absorbing it. This feature is highly appreciated in gemstones and is one of the reasons diamond is such a popular jewelry choice.
- Diamonds have a high lustre, which means they reflect light in a highly polished and glossy manner. Diamond’s smooth, glassy look is due to this characteristic, which makes it highly valued in jewelry and other decorative uses.
- Diamond is birefringent, which implies that its refractive indices differ in different directions. This characteristic can be exploited to generate optical effects such as image doubling or splitting.
| Color | Colorless, pale yellow to deep yellow, brown, white, blue-white; less commonly in oranges, pinks, greens, blues, reds, gray to black. |
| Streak | Colorless |
| Luster | Adamantine to greasy |
| Transparency | Transparent, Translucent, Opaque |
| Band gap energy | 5.5 eV |
| Cleavage | 111 perfect in four directions |
| Mohs Hardness | 10 |
| Specific Gravity | 3.52±0.01 |
| Crystal System | Isometric |
| Density | 3.5 – 3.53 g/cm3 (Measured) |
Chemical Properties of Diamond
- Diamond is exceptionally chemically stable and does not react with the majority of chemicals, including acids and bases. Because of this, it is an excellent material for use in severe or corrosive situations.
- Diamond has a low reactivity because it is a poor conductor of electricity and heat and does not react well with many other elements or compounds.
- Diamond is almost entirely made of carbon, with trace amounts of additional elements such as nitrogen and boron. Diamond’s distinctive qualities are due to its high carbon concentration, which makes it one of the most valuable and sought-after jewels in the world.
- Overall, diamond’s unique chemical qualities make it a desirable material for a variety of industrial and commercial uses, such as cutting and polishing equipment, electronics, jewelry, and scientific research.
Types of Diamonds
Natural Diamonds
The kind and quantity of impurities found in natural diamonds determine their classification.
| Types | Characteristics |
|---|---|
| Type Ia | The most common form of natural diamond, with up to 0.3% nitrogen content. |
| Type Ib | very small percentage of natural diamonds (0.1%) are of this kind, however practically all manufactured industrial diamonds are. Diamonds of type Ib can contain up to 500 ppm nitrogen. |
| Type IIa | Type IIa diamonds have so little nitrogen that it cannot be detected using infrared or ultraviolet absorption techniques. It is quite rare in nature. |
| Type IIb | This type is also quite uncommon in nature. Diamonds of type IIb contain so little nitrogen (even less than diamonds of type IIa) that the crystal is a p-type semiconductor. |
How diamonds are formed?
- Diamonds develop deep under the Earth’s mantle, some 140-190 kilometers below the surface. Diamond production is a complex process that necessitates certain pressure, temperature, and chemical composition conditions.
- Diamonds are created when carbon atoms are subjected to extreme heat and pressure. The weight of the surrounding rock and silt normally generates high pressure, while the high temperature is caused by the Earth’s interior heat.
- When carbon-rich materials, such as organic matter or carbon dioxide, are subjected to high pressure and temperature, diamond creation occurs. This leads the carbon atoms to form crystalline structures and form diamond crystals.
- Volcanic eruptions then carry these diamond crystals to the Earth’s surface. Diamonds are transported by volcanic lava, which cools and solidifies to form igneous rocks. These rocks, known as kimberlites or lamproites, contain rough diamonds.
- Diamonds can be brought to the surface through erosion and weathering of existing kimberlite pipes or alluvial deposits, in addition to volcanic eruptions. These processes gradually expose the diamond-bearing rocks and make them accessible for mining.
- Overall, diamond production is a complex process that takes millions of years deep under the Earth’s mantle. The resulting diamonds are highly coveted for their rarity, beauty, and durability, making them one of the world’s most valuable and sought-after gemstones.
Occurrence
There are several different geological environments where diamonds can be discovered worldwide
- Kimberlite pipes are volcanic tubes that carry diamonds and other minerals to the Earth’s surface. The bulk of diamonds are created in these tubes. Kimberlite pipes are often found in solid continental areas or old cratons, and they are frequently linked to deep-seated mantle sources.
- Lamproites, which resemble kimberlite but are often connected with younger, less stable geological zones, also contain diamonds.
- In alluvial deposits diamonds can be accumulated after being eroded from their original source rocks and being carried downstream by rivers and streams. There are alluvial diamond deposits in riverbeds, on beaches, and in other sedimentary areas.
- Diamonds can also be discovered in marine deposits, especially along Africa’s coasts, where they are eroded from onshore deposits and carried offshore by rivers and ocean currents.
Applications of Diamonds
This exquisite and distinctive gemstone has many applications. People prefer it in jewelry form. But they should also take into account other things as well.
Jewellery Industry
Diamond is frequently used to create jewelry, and because it is a valuable metal, diamond jewelry may be rather pricey. The crystalline element sparkles against light due to its high optical dispersion and high refractive index, making it expensive jewelry. The solitaire rings used for engagement are the most popular.
Industrial Uses
Industrial applications are where diamonds are most frequently used, outside of exquisite jewelry. Diamonds are very good for polishing, cutting, and drilling because they are very strong (scoring a 10 on the Mohs Hardness Scale). Diamond saws and drills are used in numerous industries, including the automotive, mining, and military. Drill bits and saw edges are strengthened for cutting tough materials by adding small diamond particles.
Products for beauty and cosmetics
Even in the cosmetics industry, where diamond exfoliators and facials are common, diamond is widely used. It contains diamond dust or sprinkles, which are thought to have anti-aging benefits. In order to eliminate free radicals and lessen wrinkles and fine lines, diamond penetrates deeply into the skin.
Drilling Tool
Diamonds are also linked to core drilling bits and used for drilling in manufacturing, building and construction, and engineering operations. To increase the power of the drills and saws used during the cutting process, tiny diamond particles are also added to the equipment lubricant.
Audio-devices
Industrial-grade diamonds are said by some audiophiles to enhance sound quality. Because diamonds are extremely hard, they can vibrate at high speeds without warping or losing the audio quality, which allows diamond speaker domes to create high-quality sound. Diamond record needles are also a requirement for DJ gear and high-end record players. Sapphires, which rate a 9 on the Mohs Hardness Scale, are also used as record needles and are predicted to produce music of outstanding quality for 75–100 hours.
In supercomputers
Due to its excellent heat conductivity, diamond is used in electronics and computers. It is a great heat sink that traps extra heat and prevents microelectronics from overheating because of its strong thermal resistance. The creation and application of diamond wire in the design of quantum supercomputers is the subject of extensive current research. Information-storage supercomputers also employ diamond processors.
Medical Uses
The most fascinating and enticing usage of a diamond is in medical science to treat various disorders. This serves as a poison or sickness antidote. These are employed in the elimination of cancer cells from malignant tumors.
Frequently Asked Questions (FAQ)
What is the hardness level of a diamond?
Due to its lack of free electrons in its crystal lattice, diamond is the hardest element. On the Mohr scale, it receives a score of ten.
Will diamond break if it falls?
Diamonds do not break when dropped, although they can be chipped by strong, unintentional strikes. They may also shatter if a condition known as “strain” causes pressure to build up inside the stone. Small taps cause breaking, allowing the pressure to release. Although it is quite uncommon for diamonds to break in this way, it is a truth to be aware of.
Video on Interesting Facts of Diamond
References
- Helmenstine, Anne Marie, Ph.D. “Diamond Properties & Types.” ThoughtCo, Apr. 5, 2023, thoughtco.com/diamond-properties-and-types-602111.
- https://diamondbuzz.blog/diamond-properties-and-characteristics/
- https://unacademy.com/content/jee/study-material/chemistry/physical-properties-of-diamond/
- https://sciencequery.com/structure-of-diamond/
- https://www.geeksforgeeks.org/diamond-and-graphite-structure-uses-properties-applications/
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/diamond/
- https://studymind.co.uk/questions/describe-the-structure-bonding-and-properties-of-diamond/
- https://byjus.com/jee/diamond/
- https://www.britannica.com/topic/diamond-gemstone
- https://www.diamondrocks.co.uk/magazine/what-are-the-different-uses-of-diamond/
- https://allusesof.com/earth/50-important-uses-of-diamonds/
- https://geologyscience.com/minerals/diamond/

