Dye-ligand chromatography is a purification method that provides high-purity factors and unique selectivity. Dye ligands can function as substrate analogs, forming affinity interactions with their corresponding enzymes. The process of separating chemicals from a mixture is called chromatography. Extracting particular proteins from a mixture is possible, and affinity chromatography is widely employed. Here, as the sample passes down the chromatography column, the targeted proteins are captured using ligands that bind with them.

Interesting Science Videos
What is dye-ligand chromatography?
- Dye-ligand chromatography is a type of chromatography technique used for the separation and purification of biomolecules, particularly proteins and nucleic acids.
- It depends on how the target biomolecule interacts with a particular dye molecule that has been immobilized on a solid substrate (the ligand). Affinity, ion exchange, or hydrophobic contacts are frequently the basis for this interaction.
- Dye molecules are chemically coupled to a solid support matrix, such as agarose beads or cellulose. These dye ligands possess an affinity for certain biomolecules based on specific chemical properties.
- The selectivity of the chromatographic separation is determined by the dye ligand selection, which is an important factor. Selective binding and elution are possible because different dyes have differing affinities for distinct biomolecules.
- While other substances flow through the column, biomolecules of interest attach to the dye ligand with selectivity. Changes in pH, ionic strength, or competitive displacement with a competing ligand are among the methods used to elute bound biomolecules.
- Certain proteins that are challenging to purify using traditional methods can be purified using dye ligand chromatography, which combines the high capacity and ease of ion-exchange chromatography with special selectivities.
- Dye-ligand chromatography finds applications in the purification of proteins, enzymes, antibodies, and nucleic acids from complex mixtures such as cell lysates, culture supernatants, or biological fluids.
- High selectivity, mild elution conditions that preserve biomolecule function, and scalability for large-scale purification are some of its benefits.
- However, it can have drawbacks such as the requirement to optimize binding and elution conditions for every unique application and the possible leaching of the dye ligand, which could contaminate the purified biomolecule.
Principle of dye-ligand chromatography
Dye-ligand chromatography operates on the principle of selective binding between a dye molecule immobilized on a chromatographic matrix and the target molecule of interest. The interaction between the dye and the target molecule is based on specific chemical characteristics such as charge, hydrophobicity, or stereochemistry.
Utilizing the affinity between the dye ligand and the target molecule to effectively separate and purify the mixture is the principle behind it. The target molecule is specifically captured by this affinity, which is frequently based on similar chemical characteristics, minimizing non-specific interactions.
The sample containing the target molecule is added to the chromatography column that is filled with the dye-ligand matrix during the chromatographic separation process. Other components either pass through or interact poorly with the immobilized dye, but the target molecule bonds to it specifically. Target molecule binding and elution can be adjusted by adjusting variables including pH, ionic strength, and solvent composition.
Target molecule elution is accomplished by modifying the conditions to break the binding relationship and free the target molecule from the dye-ligand matrix. Following elution, the target molecule’s pure fractions can be collected for additional study or use in other processes.
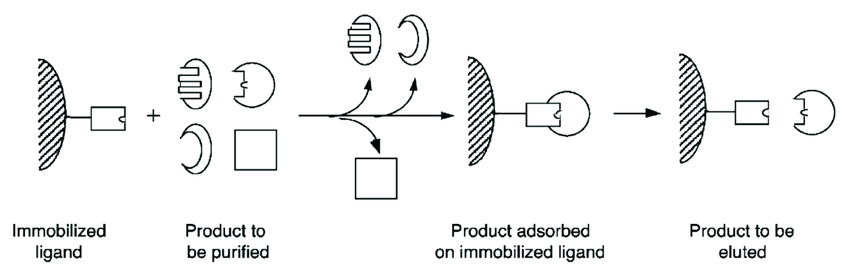
Fig: Principle of dye-ligand chromatography (Sources: https://doi.org/10.3390/s22041592)
Overall, dye-ligand chromatography provides a powerful tool for selective purification of target molecules based on their specific affinity for immobilized dye ligands, enabling efficient separation in a variety of biological and chemical applications.
Factors affecting dye-ligand chromatography
- pH: The effects of pH on elution and binding are significant. pH can be adjusted using sodium acetate (pKa 4.8), MES (pKa 6.3), phosphate (pKa 7.2), HEPES (pKa 7.6), or other suitable buffers to control the pH range for binding, which is 4 to 8. When doing batch screening, it is important to take into account the possibility of using a different buffer system to address solubility issues.
- Salts: The binding to the charged dye ligands is enhanced by low ionic strength, which is usually less than 100 mM. Substantial ionic strengths (less than 20 mM) may exacerbate issues with protein solubility. A practical elution method is to raise the ionic strength (100–1000 mM). If 1 M salt is not enough to elute the protein, the elution process requires adding solvent or detergent or adjusting the pH.
- Solvents: Dye ligand affinity for proteins is increased by hydrophobic interactions. The elution buffer strength can be increased by adding non-denaturing solvents, like glycerol or ethylene glycol, to increase the yield of protein. Use of up to 50% is possible.
- Chaotropic agents and detergents: Chaotropic substances, including urea and guanidine, can be used to improve a buffer’s elution or washing effects. One can also use non-ionic detergents like Triton X100 and Tween 80. These elements control how hydrophobically proteins bind with the dye ligand. Making sure the chosen concentration is compatible with protein activity requires careful consideration.
Dye-ligand chromatography protocol
Numerous variables, such as dye concentration, buffer conditions, flow rate, and column geometry, affect the chromatography process. There are the following steps to take:
- Purification of the dye: The dyes must first be cleaned because they are often kept in buffers that have stabilizers added and may also include other impurities. For this aim, Labrou recommended using gel filtration column chromatography.
- Immobilizing the dye onto the chromatography matrix
- The dye is adsorbed onto the matrix in the following step, which can be accomplished by a spacer molecule or by the chlorotriazine ring.
- The use of a spacer molecule, like hexamethyldiamine, can reduce steric interference from the matrix and boost dye selectivity.
- The excess dye is removed from the matrix by washing it after the dye has immobilized.
- Running the chromatogram
- After degassing, the dye-infused matrix is crammed into a chromatography column. The purpose of the degassing is to stop air bubbles from developing. Before the protein samples are loaded into the column, a buffer wash is performed.
- After the protein sample has gone through the column, any proteins that did not bind to the dye are removed from the column by giving it another wash with the buffer, and this fraction is also collected.
- The bound proteins are subsequently eluted from the column using a different buffer, and the resulting second fraction is also collected. Next, the presence or absence of the target protein(s) is/are determined for both fractions.
Dye-ligand chromatography for protein purification
The following conditions are applied for the protein purification by dye ligand chromatography that is explained below:
- Binding condition: Cell culture supernatant bound to GE Amersham Blue Sepharose 6 FF after being titrated to pH 5 with 10% acetic acid.
- Following binding, the concentration of sodium chloride stays low and the pH rises to 6.5 without eluting the protein. The following were the conditions for both the elution and the wash:
- Wash condition: 10 mM sodium phosphate, pH 6.5 (Gradient Buffer A)
- Elution condition: A linear gradient between Gradient Buffer A and Gradient Buffer B (10 mM sodium phosphate, 1 M NaCl, pH 6.5)
The protocol employed for this purification is described as follows:
- Target protein loading is employed at 0.78 mg/ml of packed resin. The target protein is placed onto a 90-ml column with 70 milligrams (17 × 2.6 cm).
- There is a 13.3 ml/min flow rate used. Based on the cross-sectional area of this column, the manufacturer would permit a flow rate as much as five times higher than this one, which is quite conservative.
- To pack the column, refer to the manufacturer’s instructions.
- Pass three column volumes of cleaning buffer (0.1 M NaOH) through the column at a rate of 13.3 ml/min to sanitize it.
- Check the pH of the eluent and equilibrate the column at 13.3 ml/min using 3 column volumes of binding buffer. Continue until the pH is right.
- Load the sample at 13.3 ml/min.
- Wash until detector baseline (usually A280 nm) is attained, using 10 column volumes of Gradient Buffer A.
- Run a linear gradient in 20 column volumes from 0 to 100% B at a rate of 13.3 ml/min. Gather fractions with a column volume of 0.5.
- Re-sanitize, then store in a bacteriostatic solution equivalent to 20% ethanol.
- Analyze fractions for activity
- Data analysis
How do dyes bind to proteins?
Numerous investigations have demonstrated that dyes, including Cibacron Blue 3-GA, attach to some enzymes’ nucleotide binding areas with ease. However, there’s always a chance that the dye will attach itself to different protein areas. Cibacron Blue 3-GA can bind non-enzymatic proteins through ionic forces, hydrophobic contacts, or exclusion-diffusion, among other ways.
- Ionic forces: This is the predominant mode of protein binding for Cibacron Blue 3-GA. Proteins with positively charged areas through the sulfonate residues or non-polar regions through the aromatic rings will bind to the dye.
- Hydrophobic interactions: It appears that ionic strength increases them while lower temperatures decrease them, and they are employed less frequently than ionic forces.
- Exclusion-diffusion: The principle of exclusion-diffusion describes how unattached proteins with a greater molecular weight are taken out of the chromatography column first, and the protein with the lowest molecular weight is eluted last. This is an uncommon occurrence. This seems to happen only at pH 9.
Application of dye-ligand chromatography
- Protein Purification: Dye-ligand chromatography is commonly used for the purification of proteins. It can selectively bind and purify target proteins based on their specific affinity for the immobilized dye ligand, allowing for efficient separation from complex mixtures.
- Enzyme Purification: Enzymes from unprocessed biological materials can be separated using dye-ligand chromatography. This method permits the selective capture and purification of highly active and pure enzymes by taking advantage of the enzyme’s affinity for the immobilized dye.
- The process of purifying viruses from cell culture supernatants or other biological materials has been achieved by the use of dye-ligand chromatography. Viral research and vaccine development as well as other downstream uses are made easier by the ability to selectively bind and purify viruses according to their unique interactions with the immobilized dye ligands.
- Antibody Purification: Dye-ligand chromatography is employed for the purification of antibodies from serum or cell culture supernatants. By leveraging the specific affinity between the antibody and the immobilized dye ligand, this technique enables the isolation of antibodies with high purity and yield for various research and diagnostic purposes.
- Peptide Purification: To separate peptides from complicated biological samples, dye-ligand chromatography is used. Efficient extraction and characterization of peptide molecules is made possible by its ability to selectively bind and purify peptides according to their unique affinity for the immobilized dye ligands.
- Nucleic Acid Purification: Nucleic acids like DNA and RNA can be made pure using dye-ligand chromatography. Applications such as PCR, sequencing, and gene expression analysis are made easier by the selective capture and purification of nucleic acids based on their unique interactions with the immobilized dye ligands.
- Drug Purification: In the pharmaceutical sector, dye-ligand chromatography is used to purify drug molecules or intermediates. High-quality pharmaceutical products can be produced by selectively capturing and purifying target chemicals according to how they interact with the immobilized dye ligands.
- Glycoprotein Purification: Glycoproteins from biological materials are extracted using dye-ligand chromatography. It facilitates glycoprotein analysis and characterization by allowing the selective binding and purification of glycoproteins based on their unique interactions with the immobilized dye ligands.
- Bioprocess Development: The purification of biopharmaceuticals is achieved by the use of dye-ligand chromatography in bioprocess development. By selectively collecting and purifying target molecules from complicated mixtures, makes purification procedures more optimized and aids in the creation of scalable and effective bioprocesses for the manufacturing of biopharmaceuticals.
- Recombinant Protein Purification: Recombinant proteins generated in different expression systems, such as bacteria, yeast, or mammalian cells, are frequently purified using dye-ligand chromatography. High-quality recombinant proteins can be produced for scientific and medicinal purposes by selectively capturing and purifying recombinant proteins according to their unique affinity for the immobilized dye ligands.
Advantages of dye-ligand chromatography
- Selective Binding: By providing selective binding between the target molecule and the immobilized dye ligand, dye-ligand chromatography enables effective separation from complex mixtures. The target molecule can be isolated with great purity thanks to its selectivity.
- Versatility: Dye-ligand chromatography can be applied to a wide range of biomolecules, including proteins, enzymes, nucleic acids, and small molecules. Its versatility makes it a valuable tool in various fields such as biochemistry, biotechnology, and pharmaceuticals.
- Increased Binding Capacity: Dye-ligand matrices frequently possess enhanced binding capabilities, facilitating the extraction of substantial quantities of target molecules within a solitary chromatographic procedure. In downstream processing, this high binding capacity improves throughput and efficiency.
- Mild Elution Conditions: Under mild elution conditions, the target molecule may usually be extracted from the dye-ligand matrix while maintaining the stability and activity of the resulting purified molecule. For delicate biomolecules like enzymes or antibodies, this is very helpful.
- Ease of Scale-up: Industrial purification procedures involving dye-ligand chromatography can be readily scaled up. Process development and optimization are made easier by the technology’s compatibility with a variety of chromatographic formats, such as packed columns and membrane chromatography.
Disadvantages of dye-ligand chromatography
- Limitations on Specificity: Although dye-ligand chromatography provides selectivity, total purification may not always be achieved due to the limited specificity of the dye ligand’s binding to the target molecule. The target molecule may nevertheless co-elute with contaminating molecules that have comparable characteristics or non-specific interactions.
- Cost: Dye-ligand chromatography matrices and ligands can be expensive compared to other chromatographic resins. The initial investment in equipment and consumables for dye-ligand chromatography may be higher, particularly for large-scale applications.
- Matrix Stability: It can be problematic if the dye-ligand matrix is unstable across multiple chromatographic cycles and under various operating circumstances. Over time, the matrix may degrade chemically or physically, changing its selectivity or binding capacity.
- Limited Ligand Availability: The availability of commercially immobilized dye ligands may be limited for certain target molecules or applications. Custom synthesis of dye ligands tailored to specific purification needs may be required, adding to the complexity and cost of the process.
- Sample Complexity: Target molecule purification from extremely complex sample matrices with a wide range of constituents may not be appropriate for dye-ligand chromatography. To simplify the sample and increase the effectiveness of purification, pre-treatment or sample preparation procedures could be required.
Despite these drawbacks, dye-ligand chromatography is still a useful method for target molecule selective purification in a range of scientific, commercial, and medical applications. For many purification problems, its benefits in terms of selectivity, adaptability, and high binding capacity make it the best option.
References
- Anne F Worrall, “Factors Effecting Dye Ligand Affinity Chromatography: A Purification Technique”, Bio Bulletin (2022), Vol. 8(4): 5-6.
- https://www.news-medical.net/life-sciences/Dye-ligand-Affinity-Chromatography-for-Protein-Purification.aspx
- Gallant, S. R., Koppaka, V., & Zecherle, N. (2008). Dye Ligand Chromatography. Affinity Chromatography, 61–70. doi:10.1007/978-1-59745-582-4_5
- Labrou, N. E. (2000). Dye-Ligand Affinity Chromatography for Protein Separation and Purification. Affinity Chromatography, 129–139. doi:10.1007/978-1-60327-261-2_13
