An electrolytic cell is crucial to the functions of daily life; they charge many electronic devices, such as phones and electric cars. Electrolytic cells use an electric current to drive a chemical reaction backward, adding potential energy to a system.
This potential energy can be used in many ways, such as reforming a solid metal from its ions, charging a battery so it can be used without remaining attached to a power source, or producing hydrogen and oxygen gas from water. Sometimes the desired outcome is to store the potential electricity to use later, like in electric cars, and sometimes it is used to obtain the product of the reverse chemical reaction, like a solid metal.
Interesting Science Videos
What is an Electrolytic Cell?
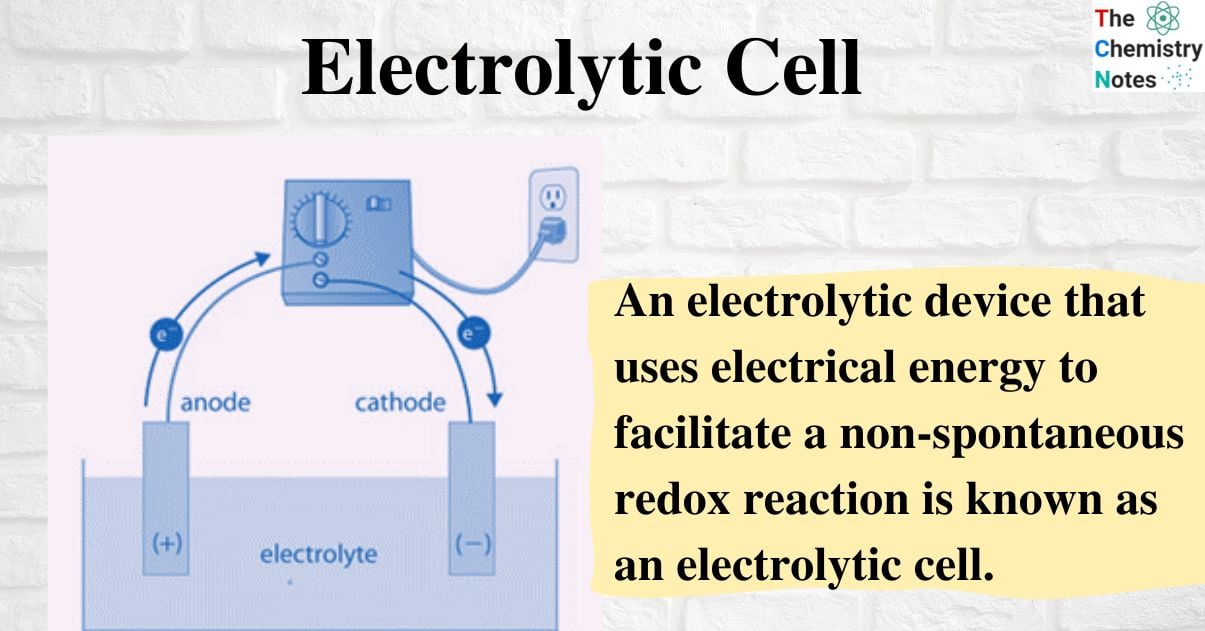
An electrolytic device that uses electrical energy to facilitate a non-spontaneous redox reaction is known as an electrolytic cell. Certain compounds can be electrolyzed using electrolytic cells, which are electrochemical cells.
For instance, water can be electrolyzed to create gaseous oxygen and gaseous hydrogen with the aid of an electrolytic cell. This is accomplished by using the flow of electrons (into the reaction environment) to break through the non-spontaneous redox reaction’s activation energy barrier.
This stands in contrast to a galvanic cell, which functions as a battery’s primary power source. In a galvanic cell, the net reaction is spontaneous, meaning that the Gibbs free energy is still negative, whereas, in an electrolytic cell, the net reaction is the opposite of this spontaneous reaction, meaning that the Gibbs free energy is positive.
Principle of Electrolytic Cell
In an electrolytic cell, a current is generated by an external voltage that flows through the cell, driving a non-spontaneous chemical reaction. An electric current flow in a galvanic cell as a result of a spontaneous chemical reaction. Between an electrolytic cell and a galvanic cell, an equilibrium electrochemical cell can be found. A counter-electromotive force precisely balances the tendency of a spontaneous reaction to push a current through the external circuit, preventing current flow. The cell changes from being a galvanic cell to being an electrolytic cell if this counter-electromotive force is increased, and vice versa.
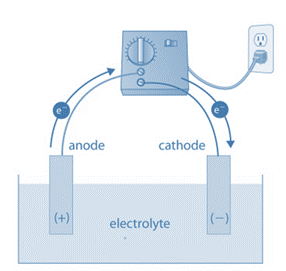
An electrolyte, two electrodes, and an electrolytic cell make up an electrolytic cell (a cathode and an anode). The electrolyte is typically a mixture of ions that have been dissolved in water or another solvent. Electrolytes can also be molten salts, like sodium chloride. The ions in the electrolyte are drawn to an electrode with the opposite charge when an external voltage is applied to the electrodes, which can then drive charge-transferring (also known as faradaic or redox) reactions. A normally stable or inert chemical compound in the solution can only be broken down by an electrolytic cell with an external electrical potential (i.e., voltage) of the right polarity and sufficient magnitude. Chemical reactions that would not naturally occur can be produced by the electrical energy used.
The cathode and anode of a galvanic cell (or battery) are positive and negative, respectively, to an external wire connected to the electrodes, forming an electric circuit. As a result, in a galvanic cell, positive electric current travels through the external circuit from the cathode to the anode.
Components of Electrolytic Cell
Several crucial components are necessary for the operation of electrolytic cells:
An electric source: It is needed because electrolytic reactions demand the introduction of energy into the system. The energy for non-lab and larger-scale electrolytic cells, such as charging a phone or electroplating, comes from the electrical grid. In experiments, this source is typically a battery.
Electrodes: The locations where the chemical reaction occurs are called electrodes. There are two electrodes, and the battery transfers electrons between the cathode, where reduction takes place, and the anode, where oxidation takes place. In contrast to oxidation, which involves the loss of electrons, the reduction is a chemical reaction that involves the addition of electrons. Redox reactions are the collective name for these simultaneous processes.
Ion solution: When electrons flow from the anode to the cathode, they must originate and terminate somewhere. At the anode, this involves either negative ions losing their charges, such as a chlorine ion losing electrons and bonding to another neutral chlorine to form chlorine gas, or positive ions gaining their charge. Alternatively, a neutrally charged molecule or atom can become positively charged, such as iron metal oxidizing to iron (II) ions. There are two options for the cathode. Positively charged ions, such as sodium ions, can gain electrons and become neutral. The second possibility is for neutral molecules/atoms to gain electrons in order to become negatively charged, such as water accepting electrons in order to form molecular hydrogen and hydroxide ions.
Working Principle of Electrolytic Cell
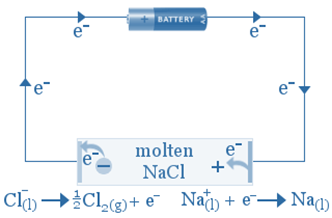
An electrolytic reaction will occur if molten NaCl(l) is placed in the container and inert electrodes of C(s) are inserted and connected to the positive and negative terminals of a battery.
Electrons from the negative terminal are directed to the cathode, where they are used to convert sodium ions into sodium atoms. As the sodium forms, it will plate onto the cathode. Sodium ions are moving toward the cathode.
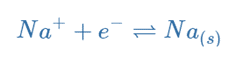
As chlorine ions oxidize to form chlorine atoms, they migrate towards the anode and release electrons. The chlorine atoms combine to form chlorine gas, which bubbles away.
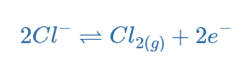
The anode is the site of oxidation, and the cathode is the site of reduction, but the charge on these two electrodes is reversed. The anode is now positively charged, while the cathode is negatively charged.
The operating conditions of the electrolyte cell are critical. The strongest reducing agent (the substance with the highest standard cell potential value in the table) will be oxidized. The substance with the highest oxidizing power will be reduced. Because hydrogen is a stronger oxidizing agent than sodium, it would undergo a reduction in the above system if an aqueous solution of sodium chloride was used.
Hence, the overall reaction is:
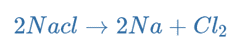
Application of Electrolytic Cell
- The primary use of electrolytic cells is to generate oxygen gas and hydrogen gas from water.
- They are also used to extract aluminum from bauxite.
- Electroplating, the process of forming a thin protective layer of a specific metal on the surface of another metal, is another notable application of electrolytic cells.
- Electrolytic cells are used in the electrorefining of many nonferrous metals.
- Electrochemical cells of this type are also used in electrowinning processes.
- It should be noted that electrolytic cells are almost always used in the industrial production of high-purity copper, high-purity zinc, and high-purity aluminum.
Examples of Electrolytic Cells
Electrolysis of Water
Electrolysis is any process in which a current is passed through an ion solution, causing reactions at the electrodes. Electrolytic cells are one method of performing electrolysis, such as water electrolysis. Individual reactions that occur during water electrolysis are listed below.
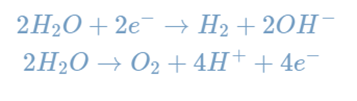
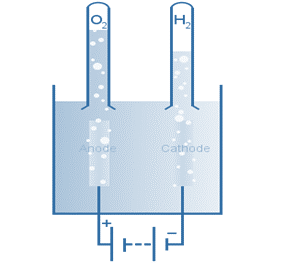
The electron flow in this electrolytic cell starts at the anode and flows to the cathode because oxidation is the loss of electrons, which occurs at the anode. As a result, oxygen gas is produced at the anode, whereas hydrogen gas is produced at the cathode.
The amount of energy required to make an electrolytic cell operate in relation to its particular reduction and oxidation reactions is known as the decomposition potential. This reaction requires approximately 1.23 volts of energy to take place; it is not spontaneous.
Since electricity does not conduct well through pure water, sodium sulfate, Na2SO4, is frequently added to the solution to transfer the charge. This electrolysis is aided by ions that flow more freely than hydrogen and hydroxide ions. These two are employed because they are significantly less likely than water to experience oxidation and reduction.
Manufacturing Sodium
Electrolysis is used to manufacture sodium metal from sodium chloride.
Recharging Batteries
Rechargeable batteries are recharged using electrolysis: rechargeable batteries operate as voltaic cells when powering devices and as electrolytic cells when being recharged.
The Edison battery, for example, is a simple, rechargeable cell invented by Thomas Edison. It is made up of two metal electrodes, one iron, and one nickel. During initial charging, a nickel oxide coating forms on the nickel electrode.
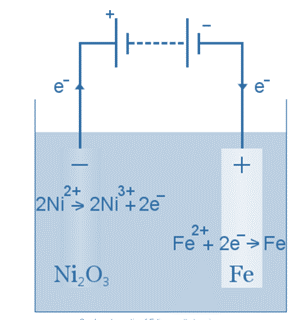
Aqueous potassium hydroxide serves as the electrolyte (the ionic liquid between the electrodes). When discharging, the Edison cell functions as a voltaic cell. When charged, the cell functions as an electrolytic cell.
References
- Skoog, Douglas A.; West, Donald M.; Holler, F. James; Crouch, Stanley R. (2014). Fundamentals of Analytical Chemistry. (9th ed.)
- Petrucci, et al. General Chemistry: Principles & Modern Applications. 9th ed. Upper Saddle River, New Jersey: Pearson/Prentice Hall, 2007.
- Kolbe, Hermann. The Electrolysis of Organic Compounds. Edinburgh : E. & S. Livingstone, 1947.
- https://byjus.com/chemistry/electrolyticcell/#:~:text=An%20electrolytic%20cell%20can%20be,the%20electrolysis%20of%20certain%20 compounds.
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/ Electrolytic_ Cells
- https://en.wikipedia.org/wiki/Electrolytic_cell#Principles
- https://study.com/learn/lesson/electrolytic-cells-overview-reduction-electron-flow.html
- https://www.chemicool.com/definition/electrolytic-cell.html
- https://www.toppr.com/ask/en-np/content/concept/electrolytic-cell-203311/
