In a multiple-bonded complex, the electromeric effect occurs when one of the attacking reagents completely transfers the pi electrons to one of the two atoms, and the other atoms are left with no pi electrons. Polarity results from the complete exchange of the paired electrons. This is most noticeable in organic molecules, which typically have either double or triple bonds.
In other words, the polarized molecule will return to its unpolarized (or “original”) form as soon as the reagent is removed. The electromeric effect can be caused by either an electrophile or a nucleophile. The electromeric effect is also called the E-effect.
Interesting Science Videos
What is Electromeric Effect?
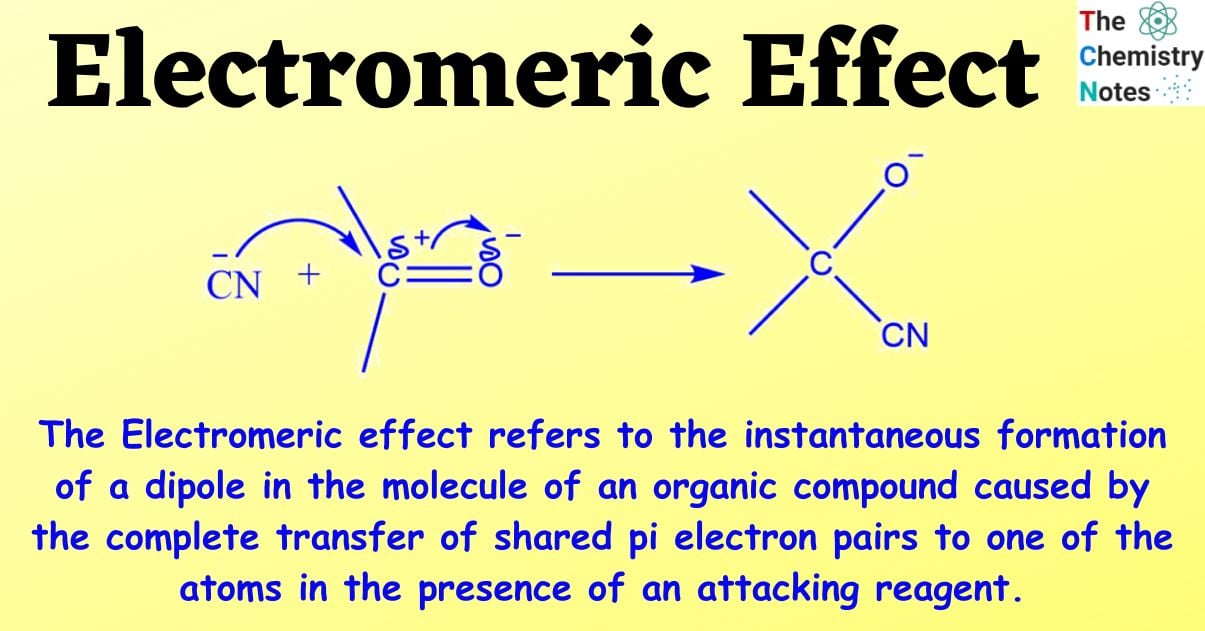
The Electromeric effect refers to the instantaneous formation of a dipole in the molecule of an organic compound caused by the complete transfer of shared pi electron pairs to one of the atoms in the presence of an attacking reagent.
At least one multiple bonds is required for organic molecules to see this effect. One pi-bonding pair of electrons is transferred fully to one of the two atoms when the atoms forming the multiple bond are subjected to an attacking reagent.
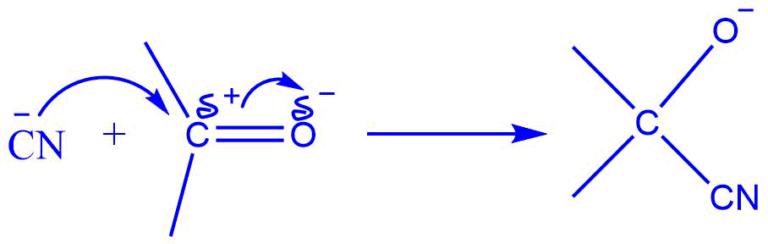
Consider an aldehyde with a carbonyl carbon; a double bond holds the carbon and oxygen atoms together.
When a negatively charged reagent assaults such a molecule, a full transfer of pi electrons from the carbon to the oxygen atom occurs. Because oxygen is more electronegative than carbon, electrons are sent towards oxygen rather than carbon.
The double bond is broken as a result of this full shift, and the negatively charged reagent now forms a connection with the positively charged carbon atom.
This transfer of electrons between atoms is known as the electromeric effect.
The electromeric effect is a short-lived phenomenon that persists for so long as the attacking reagent is in contact with the organic component. When the attacking reagent is no longer present, the polarized molecule reverts to its unaltered form.
Types of Electromeric Effect
Based on the direction of electron transfer, there are two types of Electromeric effects.
- +E effect
- -E effect
+E Effect
The movement of the electron pair of the Pi bond toward the attacking agent is called the +E effect.
The +E effect occurs when the attacking reagent is an electrophile and the pi electrons get transferred toward the positively charged atom. In other words, the presence of an electron-releasing group in the molecule, such as the methyl group (-CH3), causes electrons to be shifted away from it, which is known as the +E effect.This +E effect may be observed in the reaction of acid addition to alkenes. Protonation of ethene is an example of the positive electromeric effect.
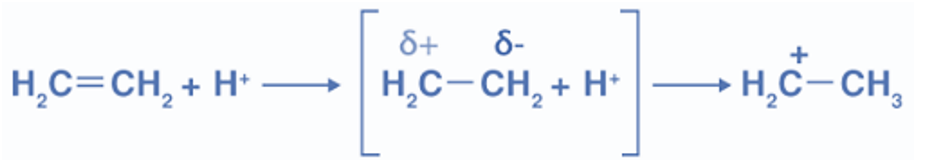
-E Effect
The movement of the electron pair of the Pi bond away from the attacking agent is called the -E effect.
When the attacking reagent is a nucleophile, the pi electrons are transferred to the nearby atom that will not form a bond with the attacking reagent. In other words, the presence of an electron-attracting group or atom-like chlorine displaces the pi – electrons towards itself, resulting in the -E effect.
The nucleophilic reagent forms a bond with the atom that has lost its pair of shared electrons.

Electromeric Effect Examples
Alkene Using Br2 in CCl4
As the reagent bromine approaches alkene, temporary polarisation occurs, with the C2 atom acquiring a negative charge and the C1 atom acquiring a positive charge. Alkenes are attacked by the electrophile Br+, resulting in the formation of a cyclic bromonium ion. Br then attacks the cyclic bromonium ion, resulting in the formation of vicinal dibromide.
Addition Of Hydrogen Halides
Hydrogen halides act simultaneously as an electrophile (proton) and a nucleophile (halide). The electrophile attacks the double bond, seizes two pi electrons, and binds them to the product molecule (carbocation). When the nucleophile (halide) forms a new molecule, the chemical reaction is complete.
Reaction of Nucleophilic Addition
When negatively-charged nucleophiles approach carbonyl molecules, the carbonyl group becomes polarized and the nucleophile attacks the positive center of the molecule.
Reaction of Electrophilic Addition
The action of electrophiles, such as H+, results in the polarization of the carbon-carbon double bond in symmetrical alkenes or alkynes.
Read Also: Inductive Effect
References
- https://www.aakash.ac.in/important-concepts/chemistry/electromeric-effect
- https://byjus.com/chemistry/electromeric-effect/
- https://unacademy.com/content/neet-ug/study-material/chemistry/a-short-note-on-electromeric-effect-and-its-significance/
- Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
