
Solvent extraction, also known as liquid-liquid extraction (LLE) and partitioning, is a technique for separating chemicals based on their relative solubilities in two immiscible liquids. Immiscible liquids are those that do not mix and split into layers when agitated together. Typically, these liquids are water and an organic solvent.
Solvent extraction is the process by which a chemical transfers from one solvent to another due to the difference in solubility or distribution coefficient between these two immiscible (or marginally soluble) solvents. It is a quantitative separation method for chemicals.
Interesting Science Videos
What is solvent extraction?
Liquid-liquid extraction is the extraction of material from one liquid phase into another. The Solvent Extraction (S.E.) procedure was initially created as an analytical chemistry tool. This method has the potential to virtually separate every metallic element in the periodic table. S.E. was originally employed to separate nuclear and rare earth elements in the 1940s. However, the availability of low-cost, effective reagents prompted the development of large-scale S.E. techniques for extracting non-ferrous metals from hydrometallurgical leach liquors.
The distribution concept is most commonly used in solvent extraction, which is commonly used in laboratories or large-scale production. Organic molecules are far more soluble in organic solvents such as benzene, chloroform, and ether than in water, while these solvents are insoluble in water.
By adding benzene, chloroform, and other solvents, organic compounds can be easily separated from the mixture with inorganic chemicals in aqueous media. When shaken, these divide into two layers. Because organic molecules have a distribution ratio that favors the benzene phase, more of them would enter into a non-aqueous layer. Finally, the non-aqueous layer is removed and the refined chemical is distilled. It is essential in organic synthesis. This technique is preferred for separating or refining organic compounds with low boiling points or that are thermally unstable.
Principle of the solvent extraction process
The principle of solvent extraction follows the Nernst distribution law or partition law, which states that when a solute particle is distributed in two solvents, it is also distributed in a fixed ratio regardless of the solute present.
If two solvents are immiscible with one another, the desired solute may be soluble in both, but the degree of solubility may differ. When solutions of a certain solute in two immiscible solvents (say, aqueous and organic solvents) are mixed, the solute distributes itself from one phase to the other in a fixed ratio. This is the fundamental principle of solvent extraction.
If x1 is the concentration of solute in Phase 1 (organic phase) and x2 is the concentration of salute in Phase 2 (aqueous phase), the distribution coefficient is given by x1/x2.
Distribution coefficient KD = x1/x2
This law provides the best outcomes when used in the following circumstances:
At the same temperature
For dilute solution.
The molecular state of the solute is the same in both phases
- It should be noted that the law makes no consideration for the actions of diverse species.
- As a result, it is only applicable for very dilute solutions where the activity ratio approaches unity.
- This Law does not apply if any of the distributing species dissociates or associates during either phase.
Distribution coefficient
Solutes frequently dissolve in part into both layers when a solution is placed in a separatory funnel and agitated with an immiscible solvent. The components are said to “partition” or “distribute themselves” between the two layers. When equilibrium is reached, the ratio of solute concentration in each layer is constant for each system, and this can be represented by a value K, which is known as the partition coefficient or distribution coefficient.
KD = Organic phase molarity / aqueous phase molarity
In practice, the solute frequently exits in two immiscible phases due to polymerization, ionization, and complexation, hence the partition coefficient is utilized to define the ratio.
Let’s use ammonia as an example, which may be dissolved in two immiscible solvents, to better understand the distribution of the solute in two solvents. Both ammonia solutions with varying ammonia concentrations are placed in a separating funnel. As solvents are immiscible, they form two distinct layers. The separating funnel is then thoroughly agitated, and the ammonia, which is soluble in both metals ents, begins to migrate across the phases to achieve equilibrium.
NH3 (aq) ⇌ NH3 (org)
KD = NH3 (org)/ NH3 (aq)
Solvent extraction method
A separating funnel is filled with the aqueous solution of the supplied solute. It is combined with the organic solvent of choice. The funnel is closed, and its contents are strongly shaken. It is then allowed to rest for a period of time. Water and organic solvent will separate into layers, and the solid or liquid solute will be transported from the aqueous layer to the organic layer since it is more soluble in an organic solvent. The solvent forms the upper layer of the funnel, while water forms the lower layer.
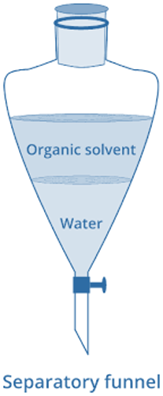
By opening the stop cock and collecting the two layers in separate bakers, the two layers can be restored. The solute can be recovered by evaporating the organic solvent.
Factors affecting solvent extraction
The parameters that contribute to a higher distribution ratio of solute to be extracted are the most important prerequisite of solvent extraction for high efficiency. It relies on the type of extractant, solvent, pH, and a number of other factors.
Solvent
It is regarded as the most significant feature in establishing a specific extraction technique for the extraction of elements. The following qualities should be present in an extraction solvent:
The solvent must possess the following characteristics.
- high extraction capacity: those with high solubility of the entrusted solute
- A sufficient density differential between the aqueous and non-aqueous phases
- In the aqueous phase, it is very immiscible.
- low viscosity
- Non-toxic
- Inexpensive
- Simple to recover
pH
The pH of a metal complex impacts its stability and charge. The pH at which the metal ion complex is the most stable and neutral is the optimal pH for metal ion extraction.
Masking agent
It is frequently difficult to separate ion pairs during metal extraction operations. Masking agents are metal complexing agents that are used to increase the separation factor. Masking agents are chemical species that prevent undesired metal ions from being extracted with metal ions of interest.
For example, under certain conditions, EDTA is the most effective masking agent for anionic compound formation with multiple metal ions.
Salting out agent
Salting out is the process of adding electrolytes to improve the extractability of complexes. When chemicals are introduced, their dielectric constant in an aqueous phase decreases, resulting in the development of ion association complexes. NaCl is a popular choice for this purpose.
Modifiers
Modifiers are compounds that, when introduced to the aqueous phase, increase the solute’s solubility in the organic solvent. In solvent extraction, high molecular weight alcohols are typically utilized as modifiers.
Backwashing
This is a critical method for quantitative element separation in batch extractions. To eliminate contaminants, the combined organic phases from several extractions (including extractant) are treated with a fresh aqueous layer.
Oxidation state
The oxidation state of a metal ion can be modified by performing a redox reaction with a suitable reagent.
Applications of solvent extraction
- Solvent extraction is used in the production of fragrances, vegetable oils, biodiesel, etc.
- Using this process, radioactive plutonium from nuclear fuel can be recovered and utilized as nuclear fuel.
- It is employed in the separation of hazardous pollutants from sludge and sediments.
- Solvent extraction is used in the pharmaceutical business to prepare microspheres.
- Solvent extraction is utilized for organic compound separation and purification.
- This technology is utilized in Parke’s lead desilverization process.
- Using liquid-liquid extraction, orange juice is also tested for the presence of organophosphorus insecticides. Before filtering, acetonitrile is mixed with a sample of orange juice. To eliminate any potential organophosphorus pesticides from the filtrate, petroleum ether is utilized.
- Solvent extraction is used for rare earth metal extraction.
- It is also used in pharmaceuticals and quality control labs to separate organic materials.
- Solvent extraction is also used to analyze plant and animal tissues and to purify chemicals.
References
- https://eduinput.com/what-is-solvent-extraction/
- https://www.embibe.com/exams/solvent-extraction/#:~:text=Ans%3A%20Liquid%2Fliquid%2C%20liquid,common%20types%20of%20solvent%20extraction.
- https://psiberg.com/solvent-extraction/
- https://agritech.tnau.ac.in/horticulture/extraction_techniques%20_medicinal_plants.pdf.
- https://www.jsscacs.edu.in/sites/default/files/Department%20Files/Solvent%20extraction_%20Study%20Material.pdf.
- https://unacademy.com/content/cbse-class-11/study-material/chemistry/solvent-extraction/.
