Fast protein liquid chromatography (FPLC, formerly known as “fast performance liquid chromatography”) is a type of medium-pressure chromatography that was developed to purify proteins with high resolution and reproducibility. This approach employs a pump to keep the flow of the mobile phase at the required rate. The composition of the eluent is altered by changing the proportions of the fluids that comprise it. The separation parameters are maintained so that when the eluent composition varies, the sample components adsorb differentially to the stationary phase. Optimizing this process results in the separation of proteins and peptides in the sample.

Fast protein liquid chromatography is commonly used in biochemistry and enzymology.
Interesting Science Videos
What is Fast Protein Liquid Chromatography?
- Fast protein liquid chromatography is defined as liquid chromatography with high loading capacity, rapid flow rates, and a biocompatible buffer system. It allows us to measure many parameters.
- Fast protein liquid chromatography is a protein-friendly and high-performance liquid chromatography method. It employs a high-resolution small-diameter stationary phase for accurate protein separation and characterization. In addition to proteins, this approach accepts biological materials such as oligonucleotides and plasmids.
- The approach includes high loading capacity, biocompatible buffer systems, quick flow rates, and stationary phases for typical chromatography modes (such as gel filtration, ion exchange, reversed phase, and affinity chromatography).
- The system provides reproducible separation by incorporating a high level of automation such as samplers, gradient program control, and peak collecting.
- Fast protein liquid chromatography allows the user to examine multiple parameters at once, including pH, conductance, and UV levels. It also allows for numerous columns to run in tandem, reducing the time required to isolate pure protein. It is most commonly employed in biochemistry and enzymology laboratories. The method can use any flow rate for the mobile phase.
- In fast protein liquid chromatography, a computer controls the solvent velocity via a software interface to maintain a consistent flow rate of solvents. The mobile phase is an aqueous buffer, and the flow velocity is maintained constant by a positive displacement pump. The buffer composition can be changed by drawing fluids from external reservoirs. The stationary phase consists of beads, often made of cross-linked agarose, packed into a cylindrical column. The eluant is fed through the detectors, which measure the salt content (by conductivity) and protein concentration (by absorbing ultraviolet light at 280nm).
Features of Fast Protein Liquid Chromatography
- The purpose of FPLC is to purify a biomolecule (biopolymer, protein, or peptide) that may be several thousand Daltons in size. The sample volume might range from around 100 mL to several liters. As is usual with biomolecules, the eluent is a buffer, which is frequently composed of two or more buffers. It is also critical that the eluent components employed be biocompatible and not react with the chemical being purified.
- The entire FLP chromatography system is frequently employed in a cold environment (or at 4°C) to ensure that the structure of the protein being purified remains intact. The process is also known as biopurification or biochromatography since the pressures utilized are frequently in the range of a few bars.
- In FPLC, the stationary phase is frequently a resin. The common FPLC processes include ion exchange, gel filtration, reversed phase, and affinity chromatography. A variety of resins are available for each of these processes. Occasionally, a mix of these modes is employed. Affinity chromatography is frequently used as the initial stage in the separation of a protein from a crude extract. To get rid of impurities, this might subsequently go via ion-exchange chromatography. In situations where obtaining a pure protein in two steps is not feasible, a third stage, like ion-change chromatography, may be employed to get the ultimate pure result.
- The combination of the mobile phase buffer and the resin type used in chromatography constitutes a crucial pairing. Moreover, the buffer may consist of a blend, comprising a running buffer and an elution buffer.
- Initially, the sample is introduced into the chromatographic column using a sample pump as the running buffer flows through. Within the column, the protein of interest selectively adheres to the resin, while other components exit the system. Alteration of the buffer gradient is achieved by introducing the elution buffer via buffer-blending valves. Gradually, as more elution buffer enters the column, the protein of interest disassociates from the resin and elutes with the buffer. A protein concentration detector confirms the presence of the protein in the eluent.
- The word “fast protein liquid chromatography” refers to a technique that quickly and finely isolates protein biomolecules from a sample combination. The method can assist in the economically viable separation of protein biomolecules, including venoms, hormones, and enzymes. This technique is preferable since no protein degradation or denaturation is seen during the process.
Principle of Fast Protein Liquid Chromatography
There are four types of fast protein liquid chromatography:
- Size exclusion chromatography
- Another name for it is gel filter chromatography. It separates molecules according to size.
- In this case, a combination of molecules is put into the chromatographic column after being dissolved in the mobile phase.
- The stationary phase in this kind of chromatography is often a bead matrix, which is a porous packing material.
- The buffer in which the sample has been dissolved and passed through the stacked matrix is known as the mobile phase.
- Larger molecules are eluted or removed from the columns while the smaller molecules are momentarily trapped by the matrix bead, which functions as a filter. Different-sized particles will elute through the stationary phase at varying rates.
- Ion exchange chromatography
- The molecules can be separated using this procedure according to their charge or polarity.
- This technique can be used to separate cations and anions.
- Ions in the solution and the ion exchange resin exchange ions in a reversible manner to cause the separation.
- Four varieties of resin exchange are described by the character: weak cation exchange resin, weak anion exchange resin, strong cation exchange resin, and strong anion exchange resin. The resin that is utilized can be synthetic, either organic inorganic, or natural, such as dolomite or zeolites. For the process, the ion exchange resin needs to be of a certain quality. It should possess enough ion exchange groups, and be chemically robust, non-reactive, and denser than water.
- Before applying the sample, the stationary phase must first reach equilibrium in this process. Elution is then completed, and the product is gathered.
- Affinity Chromatography
- Purification of samples is a common use. Based on a highly specific interaction, like the interaction between an enzyme and a substrate or an antigen and an antibody, it separates the mixture.
- In this method, the target molecule ought to be passed through a standard gel matrix with well-established characteristics.
- Purification of recombinant proteins is accomplished via affinity chromatography.
- As the mobile phase containing impurities elutes out, the target molecule is trapped in the stationary phase. After that, the stationary phase will be removed to liberate and gather the target molecules.
- Reversed-phase chromatography
- This method is alternatively referred to as adsorption chromatography.
- In this process, the attachment of a mobile solute to a fixed aromatic ligand or n-alkyl hydrocarbon takes place through hydrophobic interaction.
- The sequence of elution is reversed, with polar compounds being eluted initially, followed by the retention of non-polar compounds, hence termed reversed-phase chromatography.
Instrumentation of Fast Protein Liquid Chromatography
- In the stationary phase, the resin is usually made up of agarose beads that have been cross-linked and have different surface ligands.
- Mostly buffers and organic solvents make up the mobile phase.
- Pump: Peristaltic pumps provide a steady, regulated flow. Depending on the preparation scale, such as analytical or preparative chromatography, the flow rate is changed.
- Mixer: The pump powers and regulates the mixer. particularly crucial for creating gradients between buffer sources. Throughout the FPLC run, the mixer makes sure that the buffers are used in the proper ratio to one another.
- Injection Valve: Valves that are attached to pumps guide the buffers in the desired direction. The sample loop’s contents can be injected into a column using the Inject position.
- Column: It is big [internal diameter, mm] tubes that include gel beads or small [13–15 µ] particles. It is generally made up of Inert plastic or inert fluid surfaces such as glass, titanium, or Teflon are common materials for columns. The column is intended to function at a maximum pressure of 580 psi. Additionally, pre-packed columns are offered. When not in use, columns should be kept in a solution of 24% ethanol to water.
- Fixed volume fractionation is possible with a fraction collector.
- The purpose of the flow restrictor is to create a constant backpressure that stops air bubbles from forming after the columns in the flow cells.
- On-line Filter: Rejects sample particulates that may clog the fluidic system by generating a maximum backpressure of 0.5 MPa.
- Detection system: Refractive Index (RI) detector, conductivity detector, and UV or UV/Vis spectrophotometer depending on the properties of the target analyte.
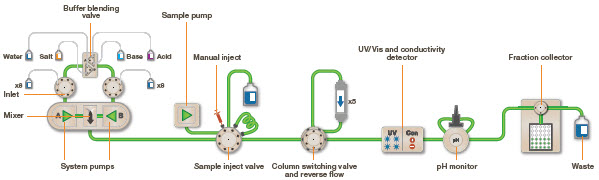
Protocol of Fast Protein Liquid Chromatography
Apparatus required
- A control unit, high-precision pumps, a column, a detection system, and a fraction collector make up the FPLC system.
- The pump makes it possible to continuously adjust the percentage of each buffer that enters the column.
- The sample solution to be injected into the column is put into the injection loop, a known-volume length of tubing.
- The injection valve, which connects the mixer and sample loop to the column, loads the sample.
- A glass or plastic cylinder filled with resin beads is the FPLC column.
- The buffer is positioned vertically, flowing downhill from top to bottom. To determine the protein concentration, the eluant from the column passes through the flow cells. The protein and salt concentrations are recorded by the detector.
Sample Preparation
- Before packing, aspirate a slurry of Sephadex G-25 resin into the column with water.
- In three to four-column volumes of buffer A (10 mM Tris-HCl, pH 7.0), equilibrate the column.
- Put the sample (50 mL) in the gravity flow container.
- After running buffer A through the column, measure A280 to keep an eye on the protein and collect it as a single, sizable peak.
- Utilizing the conductivity meter, determine the conductivity.
FPLC modes
- Ion-exchange FPLC
- Filtered and degassed buffers A (10 mM Tris–HCl, pH 7.0) and B (10 mM Tris–HCl, pH 7.0, 1 M NaCl) should be used to prime pumps A and B, respectively.
- Lower the pressure restrictions on both pumps to the minimum required for the column that is being used.
- First, use 5 volumes of buffer A and 10 volumes of buffer B to equilibrate the Mono Q column (1 mL volume). Next, use 5 volumes of buffer A.
- Use buffer A to clean the sample loading loop.
- Load the sample (0.5–10 mL; roughly 1 mg/mL) and use buffer A to clean the column.
- Gather the flow-through and test for the target protein.
- Wash the column ten times with buffer B (for regeneration) and then five times with buffer A (for regeneration).
- Scouting FPLC methods
- By altering the concentration of buffer B at various time intervals, you may produce shallowness gradients.
- Determine which column the protein binds to and use multiple buffer systems to perform chromatography at different pH values.
- Gel-filtration FPLC
- With no column in the system, prime pumps A and B with buffer C (50 mA/Tris-HCl, pH 7.0, 100 mM KCl).
- Set the P-500 pumps’ pressure restrictions.
- To equilibrate the column, connect Superose 12 and run the system at a flow rate of 0.5 mL/min for 90 minutes.
- Use buffer C to wash the sample loading loop.
- Put the sample in 200 µL at a rate of 2 mg/mL.
- Keep an eye on A280, gather peaks using the fraction collector, and note the peak’s elution volume.
How does FPLC differ from HPLC?
For the separation of small compounds, high-pressure liquid chromatography (HPLC) is frequently the preferred technique. However, biomolecule extraction and purification present difficulties for the following reasons:
- They operate in the range of temperatures that humans and other species can survive.
- They are unable to tolerate extremely high temperatures.
- In the presence of organic solvents employed in HPL, they lose either their functioning or their structure, or both.
The columns used in FPLC can only be utilized up to a maximum pressure of 3–4 MPa (435–580 psi), which is how it varies from HPLC. Glass and plastic have been used in place of stainless steel components. Since many of the resolving buffers include large concentrations of halide salts, which attack and erode stainless steel surfaces, inert surfaces are required. The solvent flow rate of an FPLC pump is between 1 and 499 ml/hr, while that of an HPLC pump is between 0.010 and 10 ml/min. Only FPLC columns can be used in an HPLC system; both can be used in an FPLC system.
Application of Fast Protein Liquid Chromatography
- The diagnosis of beta-thalassemia and the purification of animal venoms have both benefited from the application of FPLC protein profiling. Its application in detecting microvariability within a single protein is thought to be clinically significant as well.
- The nitrogenous components of beer, which are thought to contribute to foam stability, have been analyzed using FPLC quite a bit.
- The use of FPLC has made it easier to purify porphobilinogen deaminase from human erythrocytes as well as antibodies and vaccines for a variety of uses.
- Many proteins and peptides are present in bodily fluids, including plasma, urine, and cerebrospinal fluids. FPLC is a quick and accurate method for examining markers in various fluids. For example, FPLC can be used to produce a lipoprotein profile. Protein profiling in CSF fluids has been useful in identifying alterations in the fluid’s composition during various disease stages.
- FPLC is crucial in the purification of monoclonal and polyclonal antibodies from biological fluids like serum or ascites fluid. Affinity chromatography, a common FPLC technique, allows for highly specific and efficient purification of antibodies.
- Therapeutic proteins, peptides, and other biomolecules are purified using FPLC in the drug research and development process. For preclinical and clinical research, it guarantees the synthesis of pharmaceutically pure and physiologically active substances.
- FPLC techniques are applied in environmental analysis to separate and quantify proteins and peptides in environmental samples. This aids in monitoring pollution, studying ecological interactions, and assessing the impact of contaminants on ecosystems.
- Size-exclusion chromatography and affinity chromatography are two FPLC methods used to look into protein-protein interactions. This facilitates knowledge of protein function in biological systems, signaling pathways, and molecular mechanisms.
- FPLC is employed in quality control processes for monitoring the purity and consistency of protein-based pharmaceuticals, biologics, and biopharmaceuticals. It ensures compliance with regulatory standards and maintains product efficacy and safety.
Advantages of Fast Protein Liquid Chromatography
- Reproducible with excellent resolution
- The wide flow range makes it suitable for both analytical and preparative chromatography
- Very simple system programming
- Inert construction against very high salt concentrations and corrosive liquids hence columns have longer lifetime
- Since lower pressures are used in FPLC than in HPLC, a wider range of column supports is possible.
Limitations of Fast Protein Liquid Chromatography
- Glass columns are required.
- Unable to tolerate intense pressure.
- HPLC columns are not supported by the instrument.
- It’s difficult to purify thermolabile, or heat-sensitive, proteins.
References
- Walls, Dermot; Loughran, Sinéad T. (2011). [Methods in Molecular Biology] Protein Chromatography Volume 681 || Fast Protein Liquid Chromatography. , 10.1007/978-1-60761-913-0(Chapter 25), 439–447. doi:10.1007/978-1-60761-913-0_25
- https://www.bio-rad.com/en-np/applications-technologies/fast-protein-liquid-chromatography?ID=MWHBF4CZF
- https://www.news-medical.net/life-sciences/Fast-Protein-Liquid-Chromatography.aspx
- https://unacademy.com/content/kerala-psc/study-material/bioinstrumentation/fast-protein-liquid-chromatography/
- https://conductscience.com/fast-protein-liquid-chromatography-fplc-protocol/
- https://www.azolifesciences.com/article/Applications-of-Fast-Protein-Liquid-Chromatography-(FPLC).aspx
- https://courseware.cutm.ac.in/wp-content/uploads/2020/06/FPLC.pdf
