Flash chromatography is a purification technique specifically developed for rapid separation. Unlike slow and inefficient gravity-fed chromatography, flash chromatography utilizes air pressure to achieve faster and more efficient separation. The technique employed in this method deviates from the conventional column technique by utilizing silica gel particles that are slightly smaller in size, along with the application of pressurized gas at a range of 50 to 200 pounds per square inch (psi). Flash chromatography columns are commonly used in chemical separations and are designed as prepacked plastic cartridges containing silica gel particles with sizes ranging from 40 to 60 mm.
Interesting Science Videos
Flash Chromatography Theory
Chromatography is a technique that capitalizes on the variations in partitioning behavior between a mobile phase and a stationary phase in order to achieve the separation of components within a mixture. The compounds within the mixture exhibit interactions with the stationary phase that is contingent upon factors such as charge, relative solubility, or adsorption. Retention is a fundamental parameter utilized in chromatography to quantify the velocity at which a substance traverses through the chromatographic system.
In a continuous development system such as High-Performance Liquid Chromatography (HPLC) or Gas Chromatography (GC), the elution of compounds with eluents is commonly assessed by measuring their retention time (rt). Retention time refers to the duration between the injection of the sample and its subsequent detection. In a continuous development system such as thin-layer chromatography (TLC), the measurement of retention is quantified by the retention factor (Rf), which is calculated as the ratio of the distance traveled by the chemical to the distance traveled by the eluent front. The variable “Rf” represents the distance covered by the solvent front.
Principle of Flash Chromatography
The principle of flash chromatography refers to a separation technique commonly used in organic chemistry for the purification of compounds.
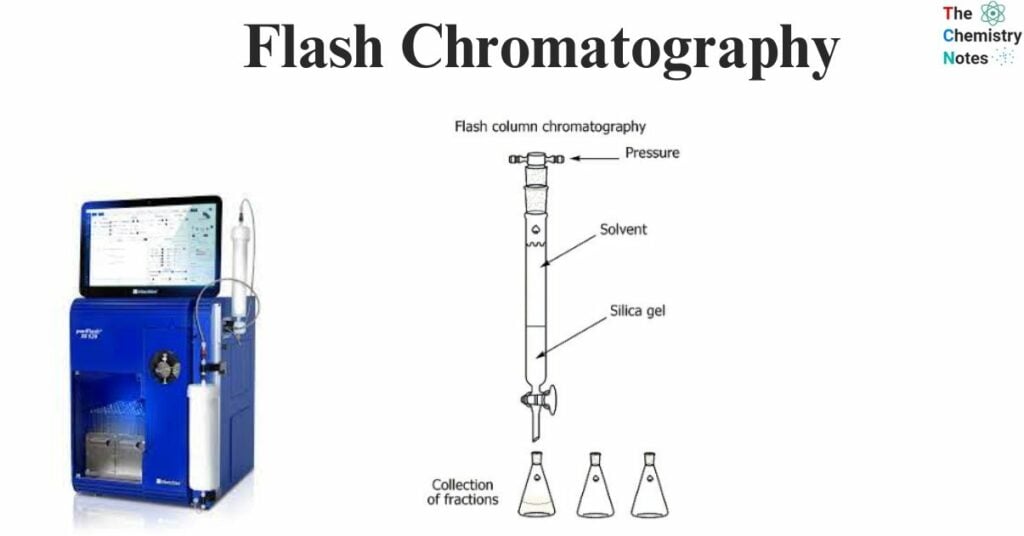
- The underlying principle involves the use of a liquid eluent, which is propelled via a brief glass column by means of gas pressure, often nitrogen or compressed air. The glass column is filled with an adsorbent material that has a specific particle size and a relatively large inner diameter. The silica gel with a particle size range of 40 – 63 μm is commonly employed as the predominant stationary phase. However, it is worth noting that alternative packing materials with different particle sizes can also be utilized.
- Particles with a size less than 25 μm are recommended to be exclusively employed in conjunction with mobile phases with extremely low viscosity. This is due to the fact that, otherwise, the flow rate would be significantly diminished. Typically, gel beds possess a height of around 15 cm and operate within a range of working pressures spanning from 1.5 to 2.0 bars.
- Initially, the sole stationary phase employed was unaltered silica, hence restricting the chromatographic technique to normal phase chromatography alone. In addition, alongside high-performance liquid chromatography (HPLC), reversed-phase materials are being employed in flash chromatography.
How does Flash Column Chromatography Work?
- A compressed gas, like air or nitrogen, or, in some cases, a pump is used instead of compressed gas to move a liquid through a column filled with a pure solid medium. This medium, which is where the mixture to be split is put, is often made of synthetic silica.
- Once the mixture is put at the top of the column, chemicals that are easier to dissolve move down the column faster than those that are harder to dissolve. Gravity helps this happen because chemicals that dissolve easily move down faster than the rest of the mixture. This makes it possible to collect these very soluble molecules.
- When these chemicals are taken out of the original mixture, they are always purer than when they were mixed together. The resultant parts come out of the bottom of the column at different times depending on how fast they diffuse. This lets scientists separate the parts of a mixture based on how well they mix together.
- Flash chromatography is basically just a faster version of regular chromatography. The method can be thought of as a hybrid because it uses both medium pressure and shorter column chromatography. It also uses air pressure to speed up the process and get the chemicals faster.
Components of Flash Chromatography
The basic prerequisite for successful separations is the choice of the proper adsorbent. The most important stationary phase in column chromatography is silica. Silica gel (SiO2) and alumina (Al2O3) are two adsorbents commonly used by the organic chemist for column chromatography. mesh or sieve through which the crude silica particle mixture is passed in the manufacturing process.
Adsorbent particle size affects how the solvent flows through the column. Smaller particles (higher mesh values) are used for flash chromatography; larger particles (lower mesh values) are used for gravity chromatography. For example, 70-230 silica gels are used for gravity columns and 230-400 mesh for flash columns. The amount of silica gel depends on the Rf difference of the compounds to be separated, and on the amount of sample.
Adsorbents
Silica: Slightly acidic medium. Best for ordinary compounds, a good separation is achieved.
Florisil: Mild, neutral medium. 200 mesh can be effective for easy separations. Less than 200 mesh is best for purification by filtration. Some compounds stick on florisil, test first.
Alumina: Basic or neutral medium. Can be effective for the easy separation, and purification of amines.
Reverse phase silica: The most polar compounds elute fastest, the most nonpolar slowest
Solvent System
Flash column chromatography is generally done with a mixture of two solvents, one of which is polar and the other not. Sometimes, you can use just one fluid. The only one-component solvent solutions that work are (from least polar to most polar):
- Pentane, petroleum ether, and hexanes are all hydrocarbons.
- The polarity of ether and dichloromethane is very close.
- Ethanoic acid.
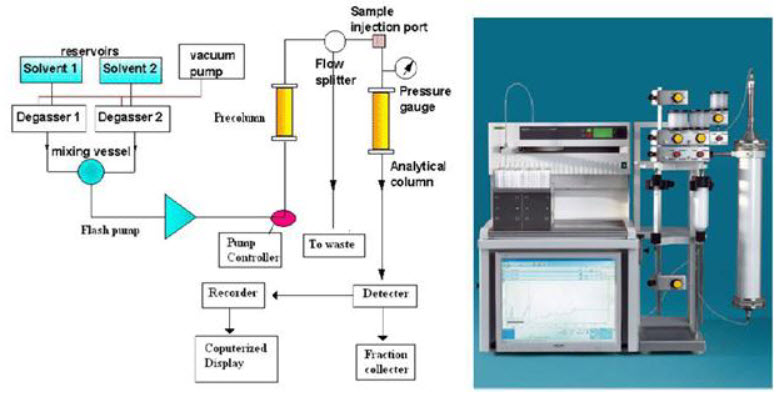
Flash Column Chromatography Instrumentation
The following are parts of general flash chromatography:
- Pump system
- Mobile phase
- Mobile phase modifier
- Stationary phase
- Columns
- Cartridges
- Detector
Pump Systems
In flash chromatography, pump systems are very important because they allow precise control of the flow rate and pressure of the liquid for the best separation results. There are different pump controllers for different separation needs and operating situations.
Pump Controller
The Pump Controller C-610 works with the Pump Module C-601 and is made for isocratic separations. With a pressure range of up to 10 bar, it makes separations that are quick and effective. The flow rate can be changed quickly with a knob, and the flow rate is shown on a large, lit LCD display on the pump controller. It also has an overpressure sensor to make sure that running is as safe as possible.
Pump Manager
The Pump Manager C-615 is a great choice for more flexible uses that involve both isocratic and gradient separations at higher pressures. It can be used quickly, is easy to program, and has a big graphical display for quick and easy settings. During a separation, the Pump Manager C-615 tells you in real-time how long the pump has been running, how much solvent it has used, and what the true pressure is. This lets you optimize the separation process. It comes with a pressure sensor and a mixing chamber, as well as input/output ports for linking two solvent valves and level sensors.
Overall, the above-mentioned pump systems and controllers give you choices for controlling flow rates, pressure, and the formation of gradients. This lets you do flash chromatography separations well. The choice of pump controller relies on how you want to separate the samples, how much pressure you need, and how automated you want the chromatography system to be.
Mobile Phase
- In chromatography, the mobile phase is an important part that helps separate mixes based on how polar they are. The type of stationary phase and the polarity of the mixture to be split determine which mobile phase to use.
- When normal-phase silica gel is used as the fixed phase, a less polar mobile phase is best. In normal-phase chromatography, solvents like dichloromethane/methane, hexane/ethyl acetate, or hexane/ether are often used.
- If reversed-phase silica gel is used as the fixed phase, on the other hand, a mobile phase with more polarity is needed. In reversed-phase chromatography, solvents like water/isopropanol or water/acetonitrile are often used.
- In addition to the mixture’s polarity, it’s also important to think about how well it dissolves. The mobile phase should be able to dissolve all of the sample parts without causing any of them to stick together. Some low-polarity mobile phases can cause sticky precipitates to form in some situations.
- Hexane (0.06), n-heptane (0.20), toluene (2.40), methyl chloride (DCM) (3.40), tetrahydrofuran (4.20), ethanol (4.30), ethyl acetate (4.30), 1-propanol (4.30), acetonitrile (6.20), methanol (6.60), and water (10.28) are commonly used as mobile phases in chromatography.
- As the mobile phase, sometimes a mixture of two liquids, one with more polarity than the other, is used to make separation easier. For example, you could use a mixture of hexane and ethyl acetate (1:1) or dichloromethane and methanol (95:5).
Mobile phase modifier
- Mobile phase modifiers are chemical reagents that are added to the mobile phase in chromatography to reduce peak tailing and improve separation resolution, especially when working with compounds that have acidic or basic groups. These modifiers interact with the silanol groups that are left on the surface of the chromatographic support. This reduces the unwanted interactions that cause peak tailing.
- Mobile phase modifiers are usually added in very small amounts, usually 1% or less, so that the makeup of the mobile phase doesn’t change too much. By adding a mobile phase modifier, the peaks become sharper and more symmetrical. This makes it easier to separate chemicals that are acidic or basic.
Some Mobile Phase Modifiers
Triethylamine: This amine-based modifier is often used to make basic molecules have less peak tailing. It helps get rid of any acidic silanol groups that are still on the stationary phase. This makes the shape of the peak better.
Acetic Acid: Acetic acid is used as a mobile phase modifier to stop acidic substances from having their peaks tail off. It helps get rid of any basic silanol groups that are still on the stationary phase, which makes the peaks more even.
Ammonium hydroxide: It is used to separate molecules that are basic. It is an alkaline mobile phase modifier. It works as a base, removing any acidic silanol groups that are still left over and cutting down on peak tailing.
Trifluoroacetic Acid: Trifluoroacetic acid is a strong acidic stabilizer that is often used in reverse-phase chromatography. It helps both acidic and basic substances have better peak shape and better resolution.
By adding these mobile phase modifiers, it can solve peak tailing problems and get better separation results, especially when working with chemicals that have acidic or basic functional groups. It is important to carefully optimize the concentration of the modifier to get the shape and resolution improvements you want without making big changes to the general makeup of the mobile phase.
Stationary phase
In chromatography, it is very important to choose the right stationary phase if you want to separate organic chemicals well. Most of what determines which stationary phase to use is how polar the molecules are and which functional groups they have. There are different kinds of stationary phases. Silica gel was the first and most popular stationary phase used in flash chromatography. Chromatographic separations have also used other stationary phases, such as reverse phase C18, alumina, and ion exchange resin.
Consideration for the Stationary Phase
Low Polarity Samples: Normal phases, reverse phases, or neutral alumina can be used as stationary phases for organic molecules with low polarity. These phases communicate with each other based on differences in polarity in order to separate well.
High Polarity Samples: C18 or cyano-based stationary phases are often used for high polarity samples. These phases have better interactions with polar functional groups, which makes it easier to separate based on changes in polarity.
Basic Functional Groups: C18, normal phases, basic alumina, or strong cation exchange (SCX) stationary phases can be used to successfully separate compounds with basic functional groups. These phases combine in specific ways with the basic molecules to make separation easier.
Acidic Functional Groups: C18, acidic alumina, neutral alumina, or strong anion exchangers are often used as stationary phases for molecules with acidic functional groups. These stages allow the acidic functional groups to interact with each other so that separation can be done well.
Acid-Sensitive Samples: For acid-sensitive samples, it is best to use a stationary phase made of neutral alumina, diol, or cyano. These stages give the acid-sensitive compounds other ways to interact that are less likely to hurt them.
Charged Samples: C18 or cyano-based stationary phases are often used when the chemicals in the sample are charged. Because of their different charges, these stages can interact with charged species and help separate them.
Columns for Flash Chromatography
Flash chromatography columns are an important part of devices that use flash chromatography to clean organic compounds. In flash chromatography, there are two main types of columns that are used: columns that are packed by hand and columns that are already packed.
Manually-packed Columns
To make manually-packed columns, good stationary phases, like silica gel or other adsorbents, are loaded into glass columns. But the packing process is done by hand, so the columns may not always be perfectly packed. If the packing isn’t done right, it can lower the sharpness and make the separations less effective. When packing by hand, you need to be skilled and pay close attention to make sure the packing is even and to avoid air holes or channels in the column.
Pre-packed Columns
Because packing by hand has its limits, you can buy columns that are already packed in different sizes. The fixed phase comes already packed with these columns, so users don’t have to pack the columns by hand. There are a few benefits to pre-packed columns over columns that are packed by hand.
Advantages of Pre-packed Column
a) Better performance: Pre-packed columns are made to have the best packing density and uniformity. This means that compounds can be purified more quickly and with better clarity. Because the packing quality is always the same, separations are more accurate and easy to repeat.
b) Saves time: Using pre-packed columns saves a lot of time because users don’t have to do the hard work of packing the columns by hand. This makes it easier to set up and run flash chromatography tests quickly.
c) Safety: Pre-packed columns are safer than columns that are packed by hand. When people pack columns by hand, they may be exposed to dangerous silica dust. This risk is taken away by pre-packed columns, making the workplace safer.
d) Productivity and Repeatability: In flash chromatography, high productivity, and repeatability are made possible by the high packing quality of pre-packed columns. Users can depend on the performance of pre-packed columns to give the same results every time they are used to purify something.
In flash chromatography, pre-packed columns make things easier, improve speed, and make things safer. They are used a lot in labs because they save time, improve productivity, and make sure that separations are accurate and repeatable.
Cartridges for Flash Chromatography
Flash chromatography cartridges are cylinders that look like pipes and are used to get the sample onto the columns in automatic flash chromatography systems. These tubes are great for samples that don’t mix well with water. There are two main kinds of cartridges on the market: solid-load cartridges that are empty and solid-load cartridges that are already packed.
Empty cartridges with solid loads
When using empty solid-load cartridges, the absorbent material can be chosen freely. Different adsorbents can be used, but silica gel is the most popular one. The following steps are required to prepare and use empty solid-load cartridges:
a) Dissolving the Sample: The sample that needs to be cleaned is mixed with a good cleaner.
b) Mixing with Silica Gel: The sample solution is mixed with silica gel powder. The bits of silica gel are spread over the sample.
c) Getting rid of the solvent: A rotary evaporator is used to get rid of the solvent, leaving the sample covered with silica gel.
d) Loading the Cartridge: The silica gel-coated sample is poured into a blank cartridge, which is then put into the flash chromatography machine. Since the cartridge is directly linked to the column, the chance of contamination is kept to a minimum.
In these empty solid-load tubes, you can use things like Celite, diatomaceous earth, or boiling chips in addition to silica gel.
Solid-load cartridges
Pre-packed solid-load cartridges are cartridges you can buy that already have a certain absorbent material in them. People who find it hard to fill empty cartridges find these cartridges easier to use. When you use pre-packed cartridges, you can add samples quickly and easily. Here are the steps you need to take to use pre-packed solid-load cartridges:
a) Dissolving the sample: The sample is mixed with a good liquid.
b) Putting Sample on Cartridge: The sample that has been dissolved is put right on the cartridge that has already been packed.
c) Absorption: The dissolved sample is taken up by the adsorbent material in the cylinder.
d) Drying: A high vacuum pump is used to dry the sample-soaked wet cartridge completely before it is put into the flash chromatography machine.
Using solid-load cartridges that have already been packed saves time and makes it easier to load samples than if empty cartridges had to be prepared first.
Overall, empty solid-load or pre-packed flash chromatography cartridges are an easy and effective way to put samples into the chromatographic system. They make it easier to clean samples with low solubility and improve the speed and accuracy of flash chromatography processes.
Detection techniques
In flash chromatography, detection methods are very important because they let chemists identify and keep track of the separated compounds. Since automation has become more common, different detectors have been added to flash chromatography devices to make the process of detecting things easier. Here are some ways that flash chromatography is often used to find things:
UV-Vis Detector
In flash chromatography, the UV-Vis detector is one of the most commonly used detectors. Many organic substances can absorb electromagnetic radiation in the ultraviolet (UV) or visible (Vis) parts of the spectrum. This feature is used by the UV-Vis detector to measure how compounds absorb light as they leave the column. It gives useful information about the presence and amount of analytes, which makes it possible to watch in real-time and collect fractions.
Refractive Index Detector
In flash chromatography, the refractive index (RI) detector is another method of measurement that is often used. It is especially useful for substances that don’t absorb much UV light or absorb little UV light. Changes in the refractive index of the chemicals that are eluting are measured by the RI detector, which sends out a signal that is proportional to their concentration. This device is especially good at finding substances that don’t react to UV light, like sugars and polymers.
Fluorescence Detector
When the compounds of interest have fluorescence qualities, the fluorescence detector is used. Some substances are fluorescent by nature or can be changed to become fluorescent. The fluorescence detector uses certain wavelengths of light to excite the eluted compounds and record the fluorescence they give off as a result. This method is good for figuring out what chemicals are fluorescent because it is sensitive and selective.
Evaporative Light Scattering Detector (ELSD)
The evaporative light scattering detector (ELSD) is a universal detecting method that can be used with any compound, no matter how well it absorbs UV light or glows in the dark. It is especially useful for finding chemicals that don’t evaporate and aren’t stable at high temperatures. The ELSD works by breaking up the eluent from the column into tiny particles and sending them into a flow of dry gas. The sample particles are evaporated, and then the light scattered by the solid particles that are left is picked up. The ELSD sends out a signal that is related to the concentration of the compounds. This makes it possible to find and measure them.
These are just a few of the ways that flash chromatography is often used to find things. Flash chromatography devices can also use other methods, such as mass spectrometry (MS) and evaporative mass detector (EMD), to help identify and describe compounds better. The type of chemicals being studied and the specific analytical needs of the study will determine which detection method to use.
Applications Of Flash Chromatography
Flash chromatography is used in many different areas, such as natural products, pharmaceuticals, carbohydrates, and lipids. Here are some specific uses for flash chromatography:
Natural Products/Nutraceuticals
- Isolation and Purification of Chromophoric and Nonchromophoric Compounds in Giant Knotweed Rhizome
- Isolation and Purification of Flavonoids from Ginkgo Biloba Leaves Extract
- Isolation and Purification of Ginsenosides from Red Panax Ginseng Extract
- Isolation and Purification of Catechins from Green Tea Extract
Carbohydrates Usage
- Conjugated Quercetin and Rutinose are cleaned up with flash chromatography.
- Lack of cleanliness Isolation of Valproic Acid from Cyclodextrin During Encapsulation:
Isolation of Aminosugar and Acarbose
- Flash chromatography is used to clean aminosugars and acarbose, which are important molecules in the field of carbohydrate chemistry.
Purification of Flavanone Glycosides
- Flash chromatography makes it possible to separate and purify flavanone glycosides, which are natural molecules with different biological functions.
Isolation of Aminoglycoside Antibiotics
- Flash chromatography is used to clean aminoglycoside antibiotics, which are important antimicrobial drugs.
Purification of Fatty Acid Methyl Esters (FAMEs)
- Flash chromatography helps clean up fatty acid methyl esters, which are often used in lipid studies and research.
Advantages of Flash Chromatography
Flash chromatography makes it possible to separate things faster than with standard column chromatography. Compounds move through a system faster when higher flow rates and pressure are used.
Cost-effective: Compared to other ways to purify, flash chromatography is usually the most cost-effective. It needs less liquid and less time to run, which makes it a good choice for large-scale purification.
Scalability: Flash chromatography is scalable, so chemists can quickly change the size of the column and how much it can hold to fit different sample sizes. It can be used for both small-scale purification in the lab and larger-scale cleaning in industry.
Ease of Use: Flash chromatography systems are easy to use because they have automatic parts that make the process of purifying easier. Software interfaces make it easy to control and monitor the system, so a bigger range of people can use it.
Flexibility: Flash chromatography lets you choose the stationary phase, the mobile phase, and the conditions for release. Based on what needs to be separated, different types of columns and stationary stages can be used.
Wide Range of Uses: Flash chromatography can be used in many different fields, such as isolating natural products, finding new drugs, making chemical compounds, and more. It works well to clean up a wide range of substances.
Disadvantages of Flash Chromatography
- Flash chromatography usually has a lower resolution than high-performance liquid chromatography (HPLC) or other advanced methods for separating substances. This can make it harder to separate complex mixtures of compounds that are closely linked.
- Flash chromatography is good for regular separations, but it may not work as well for difficult separations that require high levels of purification or selectivity.
- Flash chromatography works best for purifying samples that are between moderate and big in size. Other methods, like preparative HPLC, may be better for analyzing small sample sizes or small amounts of traces.
- Traditional flash chromatography relies mostly on UV detection, which may not work for molecules that don’t have chromophores. For substances that don’t absorb UV light, you may need to use methods like evaporative light scattering detection (ELSD) or mass spectrometry (MS) to find them.
References
- Still WC. Kahn M., Mitra A, Flash chromatography, J.Org.Chem. 43(14)1978; 2923-2925.
- A. B. Roge*, S. N. Firke, R. M. Kawade, S. K. Sarje, and S. M. Vadvalkar,BRIEF REVIEW ON: FLASH CHROMATOGRAPHY,IJPSR (2011), Vol. 2, Issue 8,1930-1937.
- William CSand Hill DC. General methods for flash chromatography using disposable column. Mol. Divers, 13(2), 2009, 247-252.
- https://microbiologynote.com/flash-chromatography-principle-instrumentation-protocol-applications/
- https://www.chromatographyonline.com/view/flash-chromatography-3
- https://www.pharmatutor.org/articles/flash-chromatography-area-applications
- https://www.biotage.com/blog/what-is-flash-chromatography-and-why-should-i-do-it#:~:text=Flash%20chromatography%20is%20a%20chemical,referred%20to%20as%20flash%20purification.

Hello Kabita, thank you for making this material available. It was really helpful.