Fluorine (F) is the heaviest of the halogen elements (Group 17; Group VIIa) and the chemical element with the highest reactivity. Even the noble gases krypton, xenon, and radon can combine with fluorine to produce compounds. Glass, metals, and even water, among other things, will burst into a brilliant flame when exposed to a concentrated stream of fluorine gas. The tiny size of its atoms and its great propensity to attract electrons (it is the most electronegative element) are responsible for its high chemical activity.
Interesting Science Videos
History of Fluorine
In 1771, Karl W. Scheele discovered fluorine. F. Henri Moissan, a French chemist, originally isolated it in 1886. In 1906, he was honored with the Nobel Prize in Chemistry.
- Fluorine-rich minerals had just a few niche applications in the past. Early scientist Georgius Agricola first documented the economic usage of fluorite, fluorine’s parent material, in the context of smelting in the 16th century.
- Agricola coined the Latin term “fluorite,” from which the English word “fluorine” was derived.
- Hydrofluoric acid was first uncovered in the late 18th century.
- Similar to chlorine, fluorine was discovered to be a bound element inside compounds by the early 19th century. Calcium fluoride was isolated from fluorite.
- Several attempts to isolate fluorine failed due to the element’s strong bonds and the toxicity of hydrogen fluoride.
- Electrolyzing a solution of potassium fluoride and hydrogen fluoride produced elemental fluorine in 1886 for the French chemist Henri Moissan, who would go on to receive the Nobel Prize in chemistry. During World War II, the Manhattan Project began mass-producing and using fluorine.
- DuPont commercialized the primary fluorochemicals around the turn of the century, including Freon refrigerant gases and polytetrafluoroethylene plastic (Teflon).
Word Origin: The Roman and French words for “flow” (fluere) inspired the naming of fluorine. Based on its occurrence in fluoric acid, Sir Humphry Davy offered the name for the element. The -ine ending follows the pattern of other halogens’ names. In Greek and Russian, however, the element is called fluor. It was originally called fluorum in scientific literature.
Occurrence of Fluorine
Fluorine can only be found in the natural world in the form of its chemical compounds, except for minute quantities of the free element that can be found in fluorspar that has been exposed to radiation from radium. It is not an uncommon element and accounts for around 0.065 percent of the crust of the earth. The most important fluorine-containing minerals are as follows:
- Fluorspar or fluorite (CaF2), which is the primary source of fluorine and may be found in deposits in the states of Illinois, Kentucky, and Derbyshire in addition to Russia, southern Germany, and the south of France.
- Cryolite (Na3AlF6), which is primarily found in Greenland;
- Fluoroapatite (Ca5[PO4]3[F,Cl]), which is widely distributed and contains variable amounts of fluorine and chlorine;
- Topaz (Al2SiO4[F,OH]2), which is the gemstone; and
- Lepidolite, which is a type of mica as well as a component of animal bones and teeth.
Fluorspar, a mineral that contains fluorine is used as a cleaning agent in several different metallurgical processes for millennia. The word “fluere,” which means “to flow,” is where we get the word “fluorspar.” After further investigation, it was determined that the mineral was a source of the element, which was eventually given the name fluorine. Fluorescence is the name given to the phenomenon in which otherwise colorless and transparent fluorspar crystals take on a bluish cast when exposed to light.
Isotopes of Fluorine and Importance
Fluorine-18 (18F), fluorine-19 (19F), and fluorine-20 (20F) are the most prevalent isotopes of fluorine.
Both 19F and 18F are often seen in nature. At least 99 percent of all fluorine in nature is found in its most prevalent isotope, 19F. Just roughly 0.01% of all fluorine occurs naturally as 18F, making it the second most common isotope.
- The most prevalent form of fluorine is 18F. With a half-life of 109 minutes, it quickly disintegrates into oxygen-18.
- 19F decays into nitrogen-19 over the course of 121 minutes.
- With a half-life of 138 minutes, 20F turns into carbon-20.
There are a variety of applications for the varied characteristics of isotopes.
- In medicine, PET scans employ 18F. In the nuclear industry, 19F is utilized for fuel enrichment. The semiconductor industry relies on 20F to fabricate silicon for use in electronic devices.
- The medical community uses 18F for PET scans. The cancer-detecting imaging modality PET scans. Once 18F has been injected into a patient, pictures may be taken. Cancer in certain organs or tissues may be shown by the photos.
- The nuclear industry uses 19F to enrich fuel. With the use of fuel enrichment, a fuel rod can contain more uranium. In turn, this increases the fuel rod’s radioactivity and the energy it can produce. The large neutron capture cross section of 19F makes it useful for uranium enrichment.
- In order to create computer chips, the semiconductor industry makes use of 20F. Silicon is the primary material used to create computer chips. Etching the silicon with 20F allows for the fabrication of chips. It’s also worth noting that solar panels employ 20F in their construction.
Allotropes of Fluorine
At ambient temperature, fluorine exists as an allotrope known as F2, however it is commonly referred to as a diatomic gas. As fluorine is an allotrope, any reference to the gaseous state is actually referring to the allotrope.
Elemental Properties of Fluorine
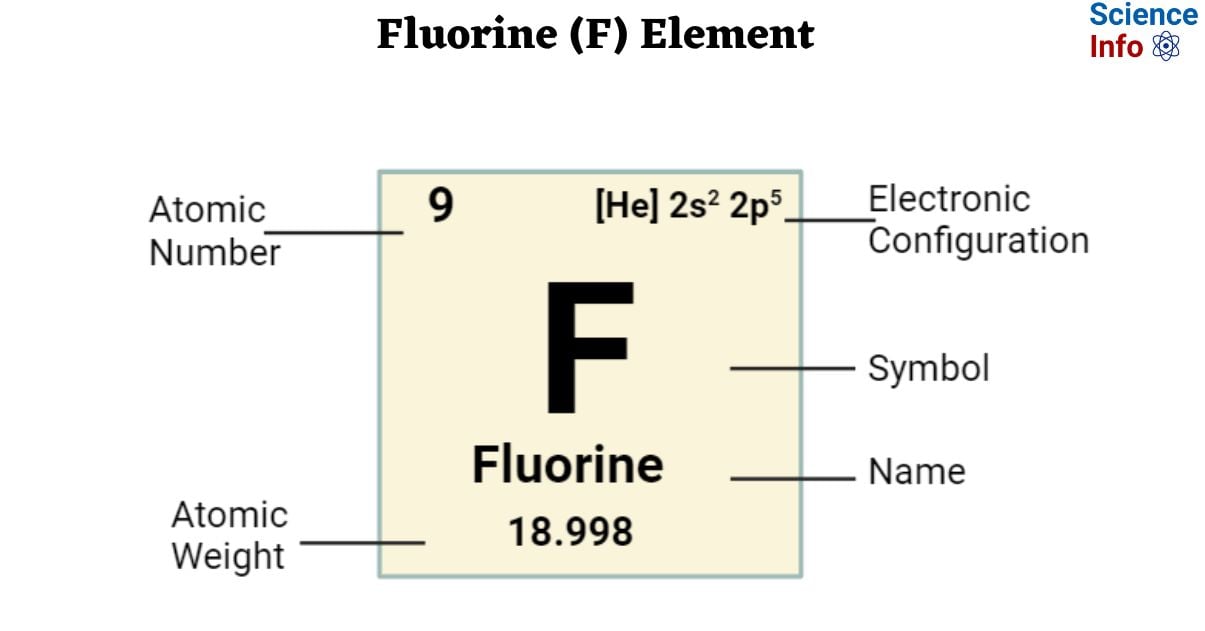
| lectronic Configuration | [He] 2s2 2p5 |
| Atomic Number | 9 |
| Atomic Weight | 18.998403 g.mol -1 |
| State at 20°C | Gas |
| Group, Period, and Block | 17, 2, p-block |
| Density | 0.001553 g cm-1 |
| Covalent radius | 64 pm |
| Van der Waals radius | 0.135 nm |
| Electron shells | 2, 7 |
Physical Properties of Fluorine
- The gaseous form of fluorine is a pale yellow color and has a strong odour.
- Natural sources of fluorine include coal, clay, and rocks.
- Combustion operations in factories produce hydrogen fluorides into the atmosphere.
- Fluorines physical state shifts from gas to liquid at temperatures as low as -188.13 ∘C. At -219.6 ∘C, liquid fluorine solidifies into a vivid yellow block. In nature, fluorine is typically found combined with other substances.
- Fluorine (the element) has an atomic weight equal to 19 g/mol, which is the same as its atomic mass. Fluoride, or F2, has an atomic weight of 38 g/mol. The chemical formula for fluoride gas, which consists of two bonded fluorine atoms, is F2.
- The oxidizing power of fluorine is unparalleled by any other element. Hence, fluoride is never found in its free state in nature since no other material can oxidize the fluoride anion to the free element.
- Industries release hydrogen fluorides into the air through combustion processes.
- Organic chloride compounds and salt spray contribute to the atmosphere’s 0.6 ppb fluorine concentration.
- The elemental form of fluorine is highly poisonous.
- The density of fluorine is 1.696 g/L at ambient temperature and pressure.
- Gaseous fluorine can cause corrosion.
- As it is not a metal, it does not carry electrical current
| Melting Point | −219.67°C, −363.41°F, 53.48 K |
| Boiling Point | −188.11°C, −306.6°F, 85.04 K |
| Density | 0.001553 g cm-1 |
| Ionization Energies | 1st: 1681 kJ/mol 2nd: 3374 kJ/mol |
| Heat of Vaporization | 6.51 kJ/mol |
| Molar Heat Capacity | Cp: 31 J/(mol·K) (at 21.1 °C) Cv: 23 J/(mol·K) (at 21.1 °C) |
| Electronegativity | Pauling scale: 3.98 |
Chemical Properties of Fluorine
- It is very electronegative. As a result, fluorine compounds are always negatively charged when mixed with other elements. Example: CF3I (trifluoroiodomethane).
- A high initial ionization enthalpy is consistent with fluorine’s extremely electronegative nature. The energy content per mole is calculated to be around 402.
- Compared to any other element, it is very small. This aids in packing several Fluorine atoms around a central coordination site. Hence, fluorine is likely to aid in the production of a wide variety of complexes (stable). Like, [SiF6]2— hexafluorosilicate.
- Both covalent and ionic fluorides of fluorine (F2) may be seen with nearly all elements except helium and neon.
- It is necessary to take extra precautions while working with fluorine because of the severe reactions it can have with organic materials like rubber, wood, and fabric.
Fluorine-air reaction
Fluorine, F2, is neither highly reactive with oxygen nor nitrogen. It interacts with the air’s moisture to produce oxygen.
2F2 (g) + 2H2O (g) → O2 (g) + 4HF (g)
Fluorine-water reaction
The reaction between F2 and water produces oxygen and ozone.
2F2 (g) + 2H2O (l) → O2 (g) + 4HF (aq)
3F2 (g) + 3H2O (l) → O3 (g) + 6HF (aq)
Fluorine-halogen reaction
At 225°C, F2 and chlorine react to generate the interhalogen species ClF. In addition to the formation of trifluoride chlorine(III) fluoride, the reaction does not proceed to completion.
Cl2 (g) + F2 (g) → 2ClF (g)
Cl2 (g) + 3F2 (g) → 2ClF3 (g)
At more demanding circumstances, excess F2 reacts with chlorine, Cl2, at 350 degrees Celsius, and 225 atmospheres of pressure to form the interhalogen species ClF5.
Cl2 (g) + 5F2 (g) → 2ClF5 (g)
In the gas phase, F2 and bromine react to generate the interhalogen species BrF. It is difficult to get a pure product because BrF disperses at room temperature into bromine, Br2, BrF3, and BrF5.
Br2 (g) + F2 (g) → 2BrF (g)
3BrF (g) → Br2 (l) + BrF3 (l)
5BrF (g) → 2Br2 (l) + BrF5 (l)
At 150 degrees Celsius, excess F2 combines with bromine, Br2, to generate the interhalogen species BrF5.
Br2 (l) + 5F2 (g) → 2BrF5 (l)
At around -45°C in a CCl3F solvent, F2, interacts with iodine, I2, to generate the interhalogen species IF. Pure product is difficult to get because, at room temperature, IF disperses into iodine, I2, and IF5.
I2 (g) + F2 (g) → 2IF (g)
5IF (g) → 2I2 (s) + IF5 (l)
Uses and Applications of Fluorine
- Atomic fluorine and molecular fluorine are utilized for plasma etching in the manufacture of semiconductors, flat panel displays, and MEMs.
- Teflon and other low-friction polymers, as well as halons like freon, make indirect use of fluorine.
- To separate uranium-235 from uranium-238 for reactor fuel, a gaseous diffusion technique is required, and uranium is used in the manufacture of uranium hexafluoride (UF6).
- Commercial production of hydrogen fluoride and boron trifluoride (BF3) is supported by their use as catalysts for the alkylation processes necessary for the synthesis of a wide variety of organic molecules.
- To lessen the chances of kids developing cavities, sodium fluoride is often added to public water supplies.
- The pharmaceutical and agricultural industries have recently emerged as the most significant users of fluorine compounds.
- The biological characteristics of these compounds are drastically altered by the selective replacement of fluorine. The usage of fluoro chloro hydrocarbons in these applications is widespread.
- The contentious addition of fluoride to municipal water sources and its use in toothpaste to reduce tooth decay are both common practices.
- Sodium aluminum fluoride (Na3AlF6) is used as an electrolyte in the electrolytic smelting of aluminum metal, and hydrogen fluoride is utilized in the manufacture of many other inorganic and organic fluorine compounds of industrial interest.
- Hydrofluoric acid, a solution of hydrogen fluoride gas in water, is widely used in industry for metal cleaning and glass polishing, frosting, and etching.
Read Also: Oxygen(O) Element: Occurrence, Properties, Toxicity, Safety
Health and Environmental Effects of Fluorine
Fluorine may be found in the environment in trace levels in all living things. Hence, individuals are exposed to fluorine through ingestion (through food and water) and exhalation (via air). All form of food contains trace amounts of fluorine.
Health Effects
- The mineral fluorine is crucial to bone health and vitality. The fluoride in toothpaste can help prevent tooth decay if used twice daily. Too much fluoride in the body can injure the teeth, bones, nerves, and muscles, and can lead to conditions like tooth decay and osteoporosis.
- Industries often emit fluorine gas. Very high levels of this gas’s toxicity have been linked to fatalities. It irritates the eyes and nose even at low dosages.
- Too much fluoride in the diet when permanent teeth are still developing under the gums can cause dental fluorosis.
- Skeletal fluorosis is quite similar to dental fluorosis, except it affects the skeleton instead of the teeth. Joint pain and stiffness are the first signs. Long-term exposure can induce skeletal changes and ligament calcification.
- Concerns have been raised concerning the effects of fluoride on the human brain during development.
Environmental Effects
The accumulation of too much fluoride in the environment causes significant threats to the health of living things, including humans, animals, and plants. This puts human health at risk, interferes with the normal growth and development of organisms, and has a deleterious effect on the food chain, disrupting the natural equilibrium of the ecosystem.
- If fluorine from the atmosphere gets into the water, it will eventually get settled in the sediment. As fluorine makes its way into soils, it forms bonds that are extremely strong with the particles that make up the soil. Fluorine cannot be broken down or consumed by the environment; it’s only possible fate is to transform.
- The fluorine that is present in soils has the potential to accumulate in plant tissue. The quantity of fluorine that plants are able to take in is contingent not only on the species of plant but also on the nature of the soil and the amount of fluorine that is present in it.
- Leave damage and stunted development can result from even minimal fluorine exposure in plants that are sensitive to the element.
- An excessive amount of fluoride slows the growth of plants and diminishes agricultural yields. This occurs whether the fluoride is absorbed by the plant’s roots from the soil or by the plant’s leaves from the environment. Corns and apricots are the ones who are most severely damaged.
- There is a possibility that fluorine will build up to dangerous levels in the bodies of animals that consume plants that contain fluorine.
Toxicity, Safety, and Precautions

Toxicity
- Potential fire hazard; oxidizer.
- It has pressurized gas within and might explode if heated.
- Very Sensitive.
- Ingestion is fatal.
- Eye and skin damage are significant consequences.
- Damages eyes severely.
- Harmful to the lungs and respiratory system.
Safety Measures
- Please seek emergency medical assistance. Get in touch with a doctor or poison control center immediately. Use plenty of water to rinse your eyes out, blinking your upper and lower eyelids occasionally. Find and take out your lenses. Keep rinsing for a minimum of 10 minutes. Chemical burns require immediate medical attention.
- Get a doctor right now. You should get in touch with a doctor or poison control center immediately. Take the sufferer outside into the fresh air and put them in a posture where they can relax and breathe easily. A mask or self-contained breathing device should be used by the rescuer if the presence of fumes is still suspected. Artificial respiration or oxygen should be administered by skilled professionals if breathing is not detected, if breathing is erratic, or if respiratory arrest occurs. Giving mouth-to-mouth resuscitation might be harmful to the rescuer. If the person is unconscious, put them in the recovery position and obtain medical help right away. Don’t close off your airway. Whether it’s the collar, the tie, the belt, or the waistline, loosen it.
- Use protective hands or wash contaminated clothing before removing it. Keep rinsing for a minimum of 10 minutes. Chemical burns require immediate medical attention. Clothing should be washed before being reused. Please clean your shoes before reusing them.
Precautions
- Wear the proper personal protection equipment. Avoid getting this substance in your eyes, on your skin, or on your clothing. Avoid breathing any gas that may be present. Warning: only use in environments with sufficient ventilation.
- Maintain a safe distance from clothes, materials that are incompatible with one another, and materials that can catch fire.
- Always make sure that the reduction valves are clean and free of oil and grease. Empty containers often still contain residue from the product they formerly contained, which poses a safety risk. Under no circumstances may the container be punctured or burned. Be careful to use equipment that is rated for the pressure in the cylinder. Always make sure the valve is closed after using it, and especially while it’s empty.
- Avoid causing any damage to the cylinders by dragging, rolling, sliding, or dropping them. Safeguard them from any potential harm. While transporting cylinders, you should make use of an appropriate hand truck.
- If a risk assessment determines that wearing gloves that are resistant to chemicals and impermeable to chemicals and that conform with a standard that has been established is essential when handling chemical goods, then these gloves should be worn at all times. During usage, check to see whether the gloves have lost any of their protective qualities, keeping in mind the specifications that were given by the maker of the gloves. It is important to keep in mind that the amount of time it takes for any particular glove material to break through may vary depending on the manufacturer of the glove. When working with mixes that are comprised of more than one material, it is impossible to provide a precise estimation of the amount of time the gloves will protect the user.
- In the event that a risk assessment reveals that it is essential for you to do so, ensure that you are wearing a respirator that is either air-fed or purifying the air that you breathe and that it complies with a recognized standard. The selection of a respirator need to be predicated on the exposure levels that are already known or that can be reasonably estimated, the dangers posed by the product in question, and the secure operational boundaries of the respirator in question.
Handling and Storage
- Only store in areas where the temperature will not go beyond 125 degrees Fahrenheit (52 degrees Celsius).
- Place signage in storage and usage areas that read “No Smoking” and “No Open Fire.” There should not be any potential ignition sources.
- Separate the packages and protect against the possibility of fire and/or explosion damage in accordance with the applicable codes and requirements (for example, in the United States, NFPA 30, NFPA 55, NFPA 70, and/or NFPA 221), or in accordance with the requirements that have been determined by the Authority Having Jurisdiction (AHJ).
- Always secure containers in the upright position to prevent them from toppling over or falling over accidentally. While the container is not being used, make sure the valve protection cap, if one was provided, is screwed on as tightly as possible by hand.
- Keep containers both filled and empty in different locations. To avoid holding full containers for extended periods, use an inventory system that operates on the “first in, first out” principle.
- Use pipe and equipment that has been sufficiently developed to be able to handle the pressures that will be experienced while working with products that are under pressure. Never attempt to work on a system that is under pressure.
- Install a device in the pipe that prevents back flow of liquid. Because of the lack of oxygen, gases can cause quick asphyxia; it is important to store and utilize them in an area with appropriate ventilation. In the event that there is a leak, the container valve should be closed, and the system should be blown down in a way that is safe for the environment and complies with all applicable international, federal/national, state/provincial, and local legislation.
- The leak should then be repaired. Never position a container in such a way that it might become a component of an electrical circuit.
References
- Public Health Statement for Fluorides, Hydrogen Fluoride, and Fluorine. CAS#: Hydrogen Fluoride 7664-39-3; Fluorine 7782-41-4; Sodium Fluoride 7681-49-4
- https://www.airgas.com/msds/001061.pdf
- https://www.sigmaaldrich.com/NP/en/sds/aldrich/128333
- Emsley, John (2011). Nature’s Building Blocks: An A–Z Guide to the Elements (2nd ed.). Oxford: Oxford University Press. ISBN 978-0-19-960563-7.
- Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (2nd ed.). Oxford: Butterworth Heinemann. ISBN 0-7506-3365-4.
- https://www.lenntech.com/periodic/elements/f.htm#:~:text=If%20fluorine%20is%20absorbed%20too,causes%20eye%20and%20nose%20irritations.
- https://www.healthline.com/nutrition/fluoride-good-or-bad#bottom-line
- Patnaik, Pradyot (2007). A Comprehensive Guide to the Hazardous Properties of Chemical Substances (3rd ed.). Hoboken: John Wiley & Sons. ISBN 978-0-471-71458-3.
- Perry, Dale L. (2011). Handbook of Inorganic Compounds (2nd ed.). Boca Raton: CRC Press. ISBN 978-1-4398-1461-1.
