Gallium is the metallic element with the atomic number 31 and is represented by the symbol ‘Ga’ in the periodic table. It is classified as a post transition and belongs to the p-block of group 13 of the periodic table. It is a silvery-white metal similar to aluminum.
The abundance in the Earth’s crust is approximately 16.9 ppm. This is similar to the lead, cobalt, and niobium crustal abundances. The Earth’s crust does not contain gallium as a free element, and the few high-content minerals, including gallite (CuGaS2), are too rare to be used as a primary source.
Interesting Science Videos
History of Gallium
- Russian chemist Dmitri Mendeleev, was the first to predict the existence of gallium in 1975, naming it “eka-aluminum” based on its position in his periodic table. Several properties of “eka-aluminum”such as its density, melting point, oxide character, and bonding in chloride were predicted Mendeleev which came close to that of gallium.
- French chemist Paul Emile Lecoq de Boisbaudran discovered gallium in 1875 using spectroscopy from its characteristics spectrum (two violet lines) in the sample of sphalerite.
- The name for the element gallium derives from the Latin word “Gallia”, meaning Gaul.
Occurrence of Gallium
- Gallium doesn’t exist in nature as a free element. Furthermore, there are no high-gallium minerals that could be used as a primary source of the element’s or its compounds’ extraction. Instead, bauxite, coal, diaspore, germanite, and sphalerite all contain trace amounts of gallium that can be found and extracted.
- The majority of gallium is extracted as a by-product of aluminum hydroxide solution from bauxite in the Bayer process.
- Gallium can also be extracted electrochemically straight from a sodium hydroxide solution.
- China, Japan, South Korea, Russia, and Ukraine ar the leading producers of gallium.
Isotopes of Gallium
There are two naturally occurring stable isotopes of gallium: 69Ga and 71Ga
Naturally occurring isotopes
| Isotope | Mass / Da | Natural abundance (atom %) |
| 69Ga | 68.925580 (3) | 60.108 (9) |
| 71Ga | 70.9247005 (25) | 39.892 (9) |
69Ga isotope is used for studies of superconductivity.
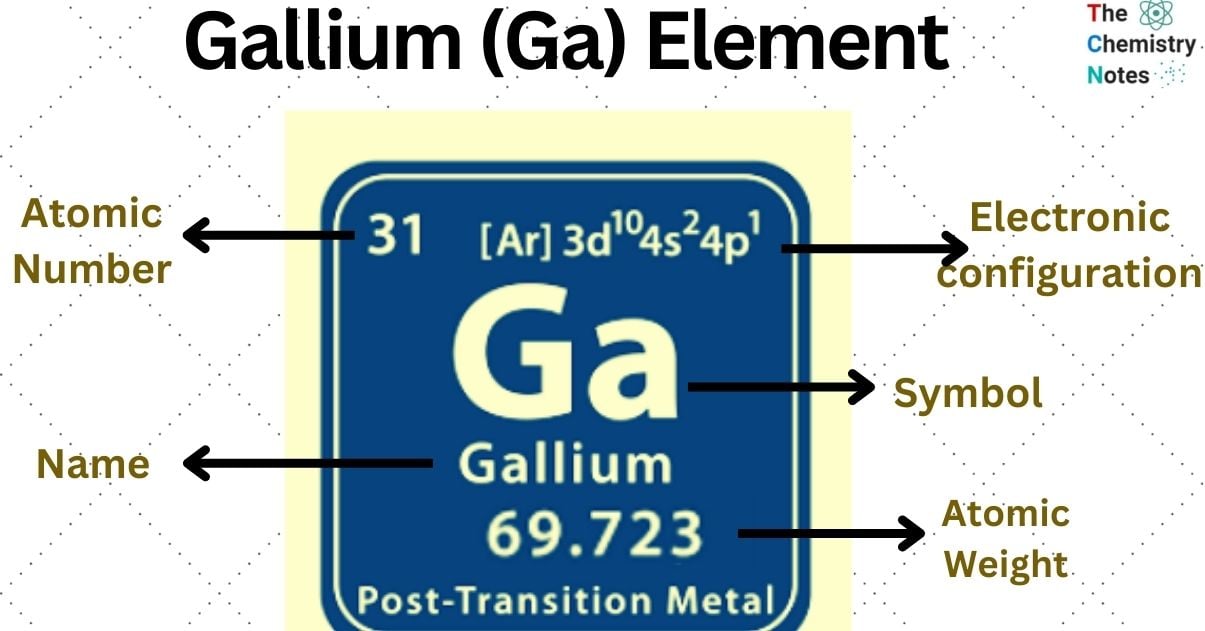
Elemental Properties of Gallium
| Electronic Configuration | [Ar] 3d10 4s2 4p1 |
| Atomic Number | 31 |
| Atomic Weight | 69.723 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 13, 4, p-block |
| Density | 5.91 g.cm -3 at 20 °C |
| Ionic radius | 0.083 nm (+3) |
| Van der Waals radius | 0.161 nm |
| Electron shells | 2, 8, 18, 3 |
| Electrons | 31 |
| Protons | 31 |
| Neutrons in most abundant isotope | 40 |
Physical Properties of Gallium
- The density of Gallium is 5.1 grams per cubic centimeter.
- Because body temperature is higher than the element’s point of melting, holding gallium in the hand will cause it to melt.
- One of the four non-radioactive metals that are liquid at ambient temperature is gallium. Rubidium, cesium, and mercury being other three.
- Galium is soft enough to be cut with a knife.
- Gallium is soluble in most acids and alkalis.
- Gallium is poorer electrical conductor than lead and is used as a semiconductor material.
- Gallium can release poisonous fumes when heated, and these gases can combine with water to generate a corrosive alkaline solution.
- Additionally, gallium has the peculiar characteristic of expanding after freezing as water.
| Color/physical appearance | silvery blue |
| Melting point/freezing point | 29.7646°C, 85.5763°F, 302.9146 K |
| Boiling point | 2229°C, 4044°F, 2502 K |
| Density | 5.91 g cm-3 at 20°C |
| Malleability | Yes |
| Ductility | Yes |
Chemical Properties of Gallium
- Chemically, gallium is a fairly reactive element. In contrast to alkali metals, it is not very reactive. Mercury, cesium, and rubidium are the four non-radioactive metals that are liquid at ambient temperature.
- Gallium is the only one of these elements which is neither extremely reactive like Caesium and Rubidium nor very toxic like Mercury.
- Gallium is somewhat stable in both water and air; however, it interacts with both alkaline and acidic solutions.
Chemical Reactions of Gallium
- Reaction of gallium with air
Gallium reacts with oxygen, O2 forming gallium(III) oxide
4 Ga(s) + 3 O2(s)  2 Ga2O3(s)
2 Ga2O3(s)
- Reaction of gallium with bases
Gallium dissolves in aqueous alkali.
- Reaction of gallium with sulfur
Gallium reacts with sulfur forming gallium sulfide
16 Ga(s) + 3 S8(s)  8 Ga2S3(s)
8 Ga2S3(s)
Uses Of Gallium
There are a numerous use of gallium that are discussed below.
Used As Semiconductors: Gallium is used to manufacture semiconductors to an extent of about 95% of its global production. Gallium of the highest purity is needed for this.Gallium can be combined with arsenic to create different materials, such as gallium arsenide (GaAs), gallium antimonide (GaSb), gallium nitride (GaN), etc.
These materials are used in computers, photovoltaic cells, transistors, aerospace power electronics, commercial wireless infrastructure, satellites, cable television transmission, infrared laser diodes.,etc
Used As Alloys: Gallium is utilized to create various alloys because of its distinctive characteristics. Particularly, it serves as the fundamental component of low melting alloys. Medicinal Thermometer High-Performance Computing Nuclear Weapons requires gallium alloys.
Used In Pharmaceuticals: Numerous gallium salts are used in pharmaceuticals. Gallium also appears to be a promising ingredient in the treatment of cancer because of its newly identified anti-cancer properties.
Health Effects Of Gallium
Gallium is a trace element that is found in very tiny amount in human body. It has not been demonstrated to be helpful for bodily function, and it is most likely only found in tiny quantities in the natural world, water, and residue on vegetables and fruits. Human contact with pure gallium poses no risk to them. However,it has a reputation for leaving a stain on the hands.
However, some gallium compounds can be extremely harmful. Gallium(III) chloride, for instance, can produce catastrophic disorders such as pulmonary edema and partial paralysis as well as throat discomfort, breathing problems, and chest pain upon acute contact.
Environmental Effects of Gallium
Gallium is utilized in semiconductors as a doping agent. Neither mining nor the use of gallium has a negative influence on the environment.
Many of these elements have little information about their environmental behavior or human health effects. This is true for indium and gallium, two crucial elements in technology. greater environmental concentrations of indium and gallium present the possibility of greater environmental exposure, albeit the degree of this exposure is unknown.
Environmental exposures may also rise as a result of metal emissions during processing, upstream mining, and smelting processes, consumer usage, or end-of-life recycling or disposal. Vulnerable populations, such as children, are particularly vulnerable to electronic trash recycling, resulting in a major environmental health gap.
Watch out the video for interesting information about Gallium.
References
- Arora, Amit (2005). Text Book Of Inorganic Chemistry. Discovery Publishing House. pp. 389–399. ISBN 978-81-8356-013-9.
- Downs, Anthony J.; Pulham, Colin R. (1994). Sykes, A. G. (ed.). Advances in Inorganic Chemistry. Vol. 41. Academic Press. pp. 198–199. ISBN 978-0-12-023641-1.
- https://www.lenntech.com/periodic/elements/ga.htm
- https://chemistrytalk.org/gallium-element/
- https://www.rsc.org/periodic-table/element/31/gallium
- Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999). Advanced Inorganic Chemistry (6th ed.). New York: John Wiley & Sons, Inc. ISBN 978-0-471-19957-1.
- Hinds, John Iredelle Dillard (1908). Inorganic Chemistry: With the Elements of Physical and Theoretical Chemistry (2nd ed.). New York: John Wiley & Sons.

