The Heck reaction is a cross-coupling reaction between an organohalide and an alkene that produces a substituted alkene with palladium as a catalyst and base. It is an effective method for forming carbon-carbon bonds. The Heck reaction generates high yields of the desired products under relatively mild conditions and can tolerate a variety of functional groups, such as nitro groups, esters, amines, and aldehydes.
Interesting Science Videos
What is Heck Reaction?
The Heck reaction (also called Mizoroki-Heck reaction) is a cross-coupling reaction between an organohalide and an alkene that produces a substituted alkene with palladium as a catalyst and base. In organic chemistry, a cross-coupling reaction is one in which two fragments are bonded together (for example, C-C bonding) using a metal catalyst. The Heck reaction is significant from a synthetic standpoint because of its great chemoselectivity and mild reaction conditions. It has low toxicity and cheaper reagent costs because the catalyst can be recycled.
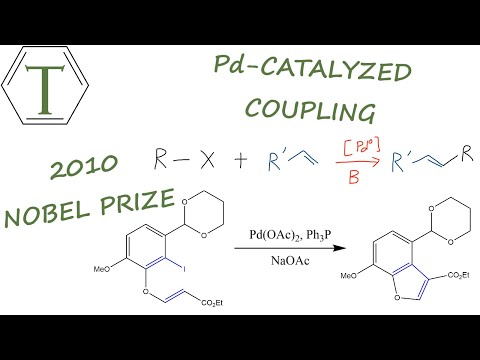
This reaction is also known as the Mizoroki-Heck reaction, after the scientists Tsutomu Mizoroki and Richard F. Heck. Richard Heck received the Nobel Prize in Chemistry in 2010 for discovering this reaction, along with Ei-ichi Negishi and Akira Suzuki.
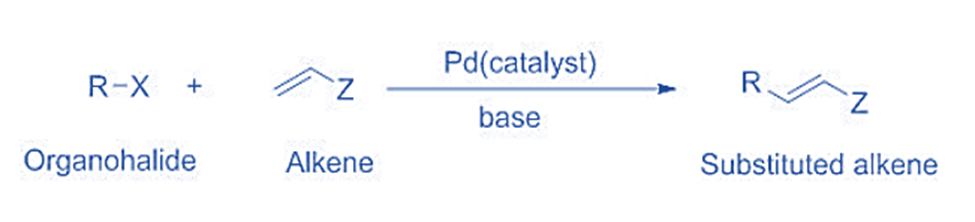
The Heck reaction was the first to produce a carbon-carbon bond following a Palladium(0)/Palladium(II) cycle. This coupling reaction may be either intramolecular or intermolecular. The reaction is commonly used to produce substituted alkenes. An example of such a reaction is shown below.
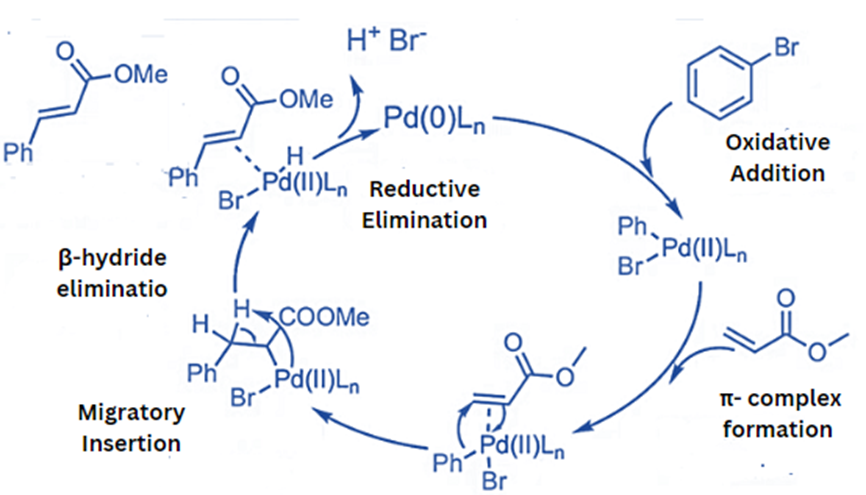
Heck Reaction Mechanism
Step 1 (Oxidative addition): In the first step, the palladium catalyst is oxidized using an aryl or vinyl halide. The insertion of palladium into the carbon-halogen bond of the halide substrate results in the production of a Pd(II) species and an aryl or vinyl Pd(II) intermediate. The alkene and palladium are then combined to form an π-complex.
Step 2 (Migratory Insertion): The alkene adds a syn to the palladium-carbon bond on the same side as the double/triple bond.
Step 3 (β-Hydride elimination): β-Hydride elimination produces a new substituted alkene. At this point, the palladium and its associated hydride must be syn-coplanar to initiate the elimination process. However, the product with Z-conformation is primarily disfavored because of steric interaction during the transition state.
Once this phase is completed, the newly generated palladium-alkene complex initiates olefin isomerization, resulting in an undesired Heck product.
These additional reactions occur because the reaction is reversible. Furthermore, this problem occurs when the rate of olefin dissociation is extremely sluggish. However, adding silver salts or bases considerably reduces the likelihood of alkene isomerization. It can be accomplished by adding reductive elimination, which results in an H-X bond.
Step 4 (Addition of Base): Upon the destruction of the new palladium-alkene π-complex, potassium carbonate reductively removes the palladium(II) component to yield the palladium(0) compound and completes the reaction.
This process produces the desired substituted alkene. Palladium is used as a catalyst throughout the reaction, resulting in stoichiometric carbonate consumption.
Application of Heck Reaction
The Heck reaction is widely employed in pharmacology, medicine, and industry because of its ability to generate large polycyclic structures. The Heck reaction can be utilized to produce:
- Isomeric 2-aryl-2,5-dihydrofuran is generated by Heck coupling aryl bromides with alkenes and using neopentyl phosphine ligands.
- Di-tert-butylneopentylphosphine (DTBNpP) is employed in the heck reaction to create 2-aryl-2,3-dihydrofuran with good selectivity, while trineopentylphosphine (TNpP) produces 2-aryl-2,5-dihydrofuran with great selectivity.
- Prosulfuron, a herbicide; Taxol, a mitotic inhibitor used in cancer chemotherapy; Singulair, an asthma medicine; and Cyclotene, a monomer for high-performance electronic resins, are all made via the same reaction mechanism.
- Indolines are very significant physiologically significant synthetic chemical compounds created through the Heck reaction.
- A variety of styrenes containing ortho-trifluoromethyl replacements.
- Chromenone derivatives include flavones and neoflavones.
- A diverse range of medications, functional materials, and bioactive natural substances.
- The Heck reaction can also be employed for producing the smoking cessation drug Chantix.
- Heck couplings between dehydrocostus lactone and aryl halides can create derivatives of guaianolide sesquiterpene lactones, which have been demonstrated to be effective in inhibiting resistant acute leukemia cells.
- The Heck reaction is used in the production of functional polymers with specific characteristics. These polymers have uses in industries such as coatings, adhesives, and electronics, where their distinct properties are advantageous.
Advantages of the Heck Reaction in C-C Bond Formation
- Palladium is highly chemoselective and resistant to organic functionality, making it suitable for usage in complex systems.

- Under mild conditions, the intramolecular Heck reaction can produce sterically hindered carbon-carbon bonds.
Differences Between Inter- and Intramolecular Heck Reactions
- In intermolecular Heck reactions, only mono- and disubstituted olefins can participate, but tri- and tetrasubstituted olefins can easily be inserted. The intramolecular Heck reaction is often more efficient than the intermolecular variant since entropy issues are eliminated.
- Electronically neutral olefins make it challenging to manage olefin addition at the intermolecular level. However, steric factors govern the unimolecular process, resulting in very selective couplings.
- Asymmetric intermolecular Heck reactions are uncommon and not applicable to all situations. Intramolecular asymmetric Heck reactions are well-developed and applicable to various substrates.
Limitations of Heck Reaction
- One downside of the heck reaction is that the Palladium catalyst will be unavailable at the end of the catalytic cycle. As a result, scientists must devise an effective method for recycling the Pd catalyst.
- Another significant disadvantage of this reaction is that the phosphine ligands associated with the palladium catalyst are both expensive and poisonous. To improve reaction efficiency, we should look for a ligand that does not contain phosphine.
Video on Heck Reaction
References
- https://www.chemistrylearner.com/heck-reaction.html
- https://testbook.com/chemistry/heck-reaction
- Jagtap, Sangeeta. 2017. “Heck Reaction—State of the Art” Catalysts 7, no. 9: 267. https://doi.org/10.3390/catal7090267
- https://byjus.com/chemistry/heck-reaction/
- https://www.vedantu.com/chemistry/heck-reaction
- https://macmillan.princeton.edu/wp-content/uploads/heckgrpmeeting.pdf
- https://www.name-reaction.com/heck-reaction