HSAB principle (i.e Hard and Soft Acids and Bases principle) was introduced by Ralph Pearson to explain the stability of metal complexes and the process of their reactions. R. G. Pearson extended and modified the qualitative relationship between Lewis acids and Lewis bases in 1963 by categorizing them as hard and soft acids and bases.
Interesting Science Videos
Features of hard and soft acids and bases
Hard acids
Hard acids are any species in which the acceptor atom has the following characteristics:
- Small size
- High polarisibility
- High charge
- Empty valence shell ordital
- E.g., H+, Li+, Ca 2+, Fe 3+
Soft acids
Soft acids are any species in which the acceptor atom has the following characteristics:
- Large size
- Low polarisibility
- low positive charge
- completely filled atomic orbitals
- E.g., Cu+, Ag+, Hg+, Hg2+
Hard bases
Hard bases are any species in which the donar atom has the following characteristics:
- Have Small ionic radii.
- High electronegativity
- Weakly polarizable
- The presence of a filled orbital or empty orbital may exist at a higher energy level.
- E.g., H2O, NH3, F–, Cl–
Soft bases
Soft bases are any species in which the donar atom has the following characteristics:
- Large size
- Low electronegativity
- Highly polarizable
- The presence of a partially filled orbital or empty orbital may exist at the low energy level.
- E.g., I–, CN–, S2-, SCN–
HSAB principle
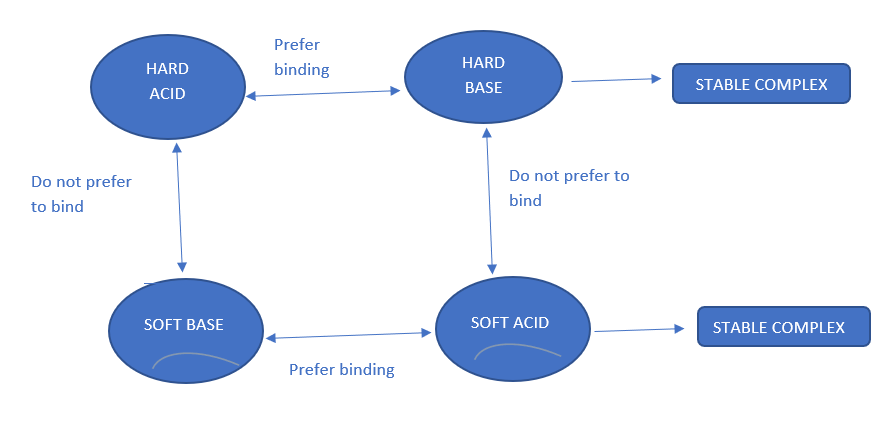
The HSAB principle states that hard acids prefer to combine with hard bases and soft acids prefer to combine with soft bases to generate stable complexes. The interaction between hard acid and a hard base produces ionic complexes while soft acid soft base interaction produces covalent complexes.
The significant electronegativity differences between hard acids and hard bases result in strong ionic interactions, whereas the electronegativities of soft acids and soft bases are almost similar, resulting in less ionic interactions. In other words, Soft acid soft base interaction produces are more covalent acid – base complexes.
Most of the time, polar covalent interactions between hard acid and soft base and soft acid and hard base are more reactive or less stable. If polar covalent molecules are allowed to react, they rapidly produce more ionic or more covalent compounds.
Hard acid + Hard base → Stable complex
Soft acid + Soft base → Stable complex
Hard acid + Soft base → less stable complex
The theoretical basis of hardness and softness
The stability of complexes generated by hard-hard and soft-soft interactions has been explained by several theories. Some of them are;
a. Ionic and covalent bond theory
This theory states that the interaction of hard acids and hard bases results in the formation of an ionic bond. The electrostatic attraction of two small oppositely charged ions leads to the production of a very stable molecule. While soft acids and soft bases interact to form a covalent bond.
b. pi- Bonding theory
Mulliken (1955) and Chatt (1956) proposed this hypothesis to explain soft-soft interaction based on pi-bonding. Soft acids have a high number of d electrons and a low oxidation state. As a result, they have a high propensity to form pi-bonds with soft bases that are also good-bonding ligands. The polarization of soft acids and bases also favors pi-bonding.
c. Pitzer’s Theory
According to Pitzer, London dispersion energies stabilize a connection between two large polarizable atoms. This energy rises as size and polarizability increase. This is why interactions between soft acid and hard bases are more stable than soft- hard acid-base interactions.
Applications of HSAB Principle
I. To predict the path of reactions
for example:
i) H+ + CH3HgOH → H2O + CH3Hg+
ii) H+ +CH3HgSH → H2 S+CH3Hg+
in this above reaction, the reaction between H+ and CH3HgOH proceeds in the forward direction as the hard acid H+ prefers to react with hard base OH– to produce H2O. On other hand, the reaction between H+ and CH3HgSH is preferred to the left, where soft base SH is likely to remain with soft acid CH3Hg+ rather than combining with hard acid H+.
II. In hydrogen bonding
Strong hydrogen bonds are feasible in the cases of H2O, NH3, and HF as the donor atoms (F, O, and N) are hard bases and their interactions with partially positively charged H i.e hard acid, are stronger.
III. To predict the stability of complexes in aqueous solutions
According to HSAB principle, [Cd(CN4)]-2 is more stable than [Cd(NH3)]-4. The HSAB principle states that soft acid favors combining with soft base and hard acid prefer to bind with hard base. Hence, the soft acid Cd2+ will favorbinds with a soft base.
IV. Analysis of basic chemical accept
a. Metals’ catalytic power can be explained by the fact that soft metal atoms easily adsorb soft bases on their surface.
b. Solubilities can be understood by the fact that hard solvents like to dissolve hard solutes, whilst soft solvents prefer to dissolve soft solutes, for example, Hg(OH)2 dissolves in an acidified aqueous solvent, but HgS does not.
V. Au Recovery
In mining operations, the softest metal ion Au+(aq) is recovered by suspending it in a diluted solution of CN-.
4 Au(s) + 8 CN– (aq) + O2 (g) + 2 H2O → 4 [Au(CN)2]– (aq) + 4 OH–
VI. Some other applications
By using the HSAB principle, it is possible to explain the existence of some metal ores. In nature, hard acids such as Mg 2+, Ca 2+, and Al 3+ are found as MgCO3, CaCO3, and AI2O3, but not as MgS, CaS or AI2 S. As CO32- and O 2- are hard bases they like to combine with hard acids to produce carbonate. Whereas, Cu+ , Ag+ , Hg+ occur in nature as sulphides.
Limitations of HSAB principle
- The significant disadvantage of the HSAB principle is that it lacks a straightforward quantitative scale for measuring acid-base strength.
- The intrinsic acid-base strengths are not taken into account, for example, OH– and F– ions are both hard bases, with OH– being about 1013 times stronger than F – ions. The relationship between hardness and natural acid-base strength has yet to be established.
- The interpretation of different reactions by dividing the participants into acid-base fragments is quite arbitrary.
suggested video
References
- https://www.shahucollegelatur.org.in/Department/Studymaterial/sci/chem/hs.pdf.
- http://www.adichemistry.com/inorganic/cochem/hsab/hard-soft-acid-base-theory.
- https://chem.libretexts.org/Courses/Lafayette_College/CHEM_212_213%3A_Inorganic_Chemistry_(Nataro)/04%3A_Acid-Base_Theories/4.04%3A_Hard-soft_Acids_and_Bases.
- https://www.khanacademy.org/test-prep/mcat/physical-sciences-practice/physical-sciences-practice-tut/e/applications-of-hard-soft-acid-base-theory.
- http://ppup.ac.in/download/econtent/pdf/HSAB%20concept%20F.pdf.
- https://psiberg.com/hsab-theory/.
