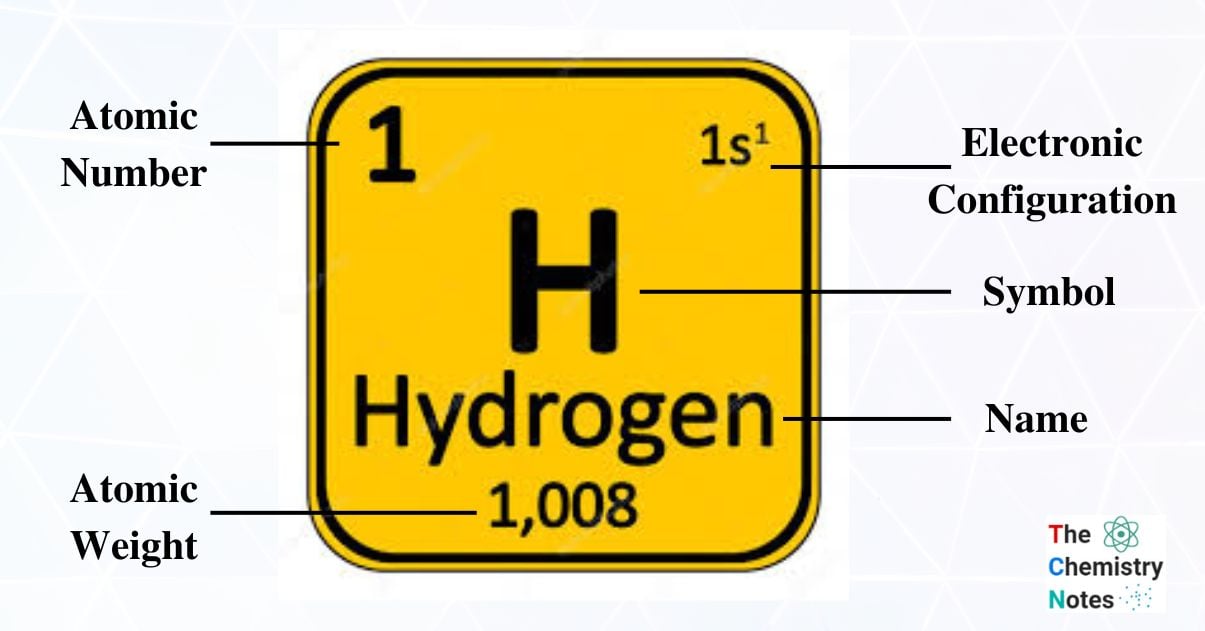
Hydrogen, with the chemical symbol H, is the first element we encounter in the periodic table of elements. It is the first and most fundamental element in the universe. Moreover, hydrogen makes up 90% of all atoms in the universe and is the lightest element in the periodic table.
Interesting Science Videos
History of Hydrogen
Theophrastus von Hohenheim, also known as Paracelsus, a German-Swiss scientist, described an ascending gas that was most likely hydrogen in 1520. However, he did not name it and discovered nothing about its properties.
Robert Boyle, an Irish alchemist, was the first to identify a flammable gas that was released when iron and dilute acid combined around 1670.
British scientist Henry Cavendish discovered hydrogen gas in 1776 by reacting zinc metal with hydrochloric acid, which led to the discovery of hydrogen as a distinct element.

( Discovered Hydrogen)
Cavendish used a spark to produce water in a demonstration to the Royal Society of London. This discovery led to his subsequent discovery that water (H2O) is composed of hydrogen and oxygen.
Building on Cavendish’s discoveries, French chemist Antoine Lavoisier named hydrogen, which was derived from the Greek words “hydro” and “genes,” which mean “water” and “born of.”
Abundance of Hydrogen
Despite being the most abundant element in the universe (three times more abundant than helium, the next most abundant element), hydrogen accounts for only about 0.14 percent of Earth’s crust by weight. The water in oceans, ice caps, rivers, lakes, and the atmosphere all contain significant amounts of it. Hydrogen is present in all animal and vegetable tissue, as well as in petroleum, as a component of numerous carbon compounds.
Even though it is common that carbon has more known compounds than any other element, the fact is that hydrogen is present in almost all carbon compounds and forms a plethora of compounds with all other elements (except for some noble gases, hydrogen compounds may be more numerous.
The majority of H2 formation is by combining natural gas and steam to create syngas (a mixture of hydrogen and carbon monoxide). It is extracted from the syngas. The electrolysis of water can also produce H2.
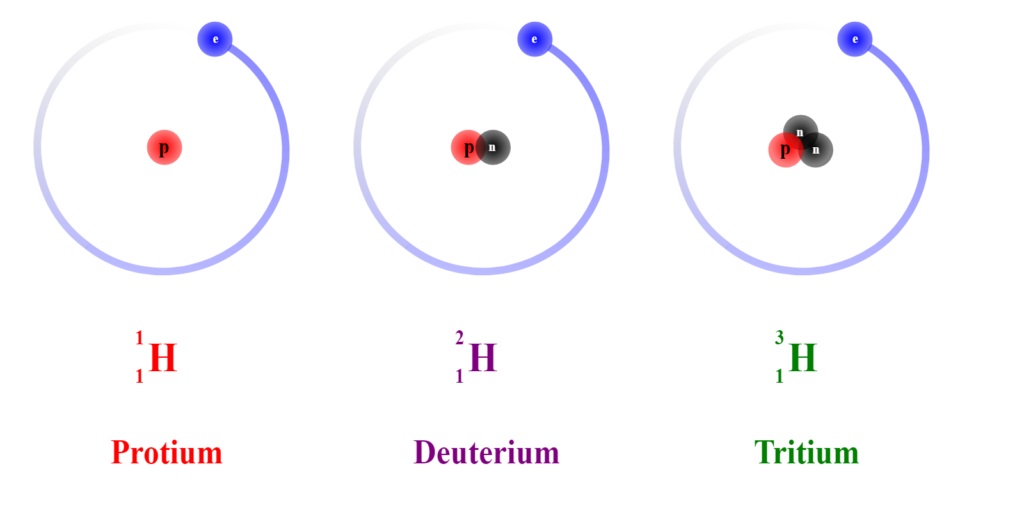
Isotope of Hydrogen
Protium ( 1H )
It is one of the most common hydrogen isotopes. It is abundant in nature, with a 99.98% abundance. One reason for this is that the nucleus of this isotope is made up of a single proton.
Deuterium ( 2H)
Its nucleus contains one proton and one neutron. It is not a radioactive substance. Heavy water is enriched with molecules containing deuterium rather than protium. It is used as a coolant as well as a neutron moderator.
Tritium ( 3H )
Its nucleus is made up of two neutrons and one proton. They are also released in trace amounts during nuclear weapons tests. It is radioactive, decaying into helium 3 via beta decay.
Physical Properties of Hydrogen
- It is a colorless and odorless gas with the lowest density of all gases. Hydrogen can be found in almost all living things, molecules, and water.
- It is present in the atmosphere as a gas in one part per million volumes.
- Also it is present in the atmosphere as a gas in one part per million volumes.
- It is spotless, non-toxic, and safe to produce, transport, and store in large quantities from various sources.
| Electronic Configuration | 1s1 |
| Block, Period and Group in periodic table | s-block, Group-1, Period-1 |
| Atomic Number | 1 |
| Atomic Weight | 1.008 gmol-1 |
| State at 20 °C | Gas |
| Melting Point | −259.16°C, −434.49°F, 13.99 K |
| Boiling Point | −252.879°C, −423.182°F, 20.271 K |
| Density | 0.000082 g/cm3 |
| VanderWaals Radius | 120 nM |
| Ionic Radius | 0.208 nM |
| Electronegativity | 2.1 (Pauling Scale) |
| Energy of first ionisation | 1311 kJ mol -1 |
| Oxidation States | -1, +1 |
| Crystal Structure | Hexagonal |
| Magnetic Ordering | Diamagnetic |
| Discovery | Henry Cavendish (1766) |
| Named By | Antoine Lavoisier (1783) |
Reactions of Hydrogen
Hydrogen halides are formed when H2 reacts with halogen.
H2 (g) + X2 (g) → 2HX (g)
When H2 reacts with the oxygen molecule it forms water. This is a highly exothermic reaction.
2H2 (g) + O2 (g) → 2H2O (l) ; Δ H= −285.9kJmol−1
It reacts with various metals to form its hydrides.
H2 (g) + 2M (g) → 2MH (s)
where M represents the alkali metals
Uses of Hydrogen
- The most important application of hydrogen is the synthesis of ammonia.
- The use of hydrogen in fuel refinement, such as hydrogen breaking down (hydrocracking) and sulfur elimination, is rapidly expanding.
- Huge amounts of hydrogen are used as rocket fuels, in conjunction with oxygen or fluorine, and as a nuclear-powered rocket propellant.
- Hydrogen fuel cells are considered a means of generating electricity, and research is being conducted on hydrogen as a possible major future fuel.
- The catalytic hydrogenation of unsaturated vegetable oils to obtain solid fat consumes a large amount of hydrogen.
- Hydrogenation process supports the production of organic chemicals.
- It can, for example, be converted to and from electricity from biofuels, as well as from natural gas and diesel fuel, with no CO2 or toxic chemical emissions.
- Another growing application for hydrogen is the direct reduction of iron ores to metallic iron, as well as the reduction of tungsten and molybdenum oxides to metals.
- In the pouring of special castings, the manufacture of magnesium, metal annealing, and the cooling of large electric motors, hydrogen (reducing) atmosphere is used.
- It was once used to inflate lighter-than-air vessels such as dirigibles and balloons, but helium is now more commonly used due to its nonflammability.
- In the laboratory, liquid hydrogen aids to generate low temperatures.
Health Effects of Hydrogen
Because of nature, all fuels pose some level of risk. The safe use of any fuel is on avoiding situations involving the three combustion factors—ignition source (spark or heat), oxidant (air), and fuel. We can design fuel systems with appropriate engineering controls and establish guidelines to enable the safe handling and use of fuel if we have a thorough understanding of its properties.
A number of hydrogen’s properties make it safer to handle and use than today’s fuels. Hydrogen, is non-toxic.
In addition, because hydrogen is much lighter than air, it dissipates rapidly when it is released, allowing for relatively rapid dispersal of the fuel in case of a leak.
High levels of this gas can create an oxygen-deficient environment. Individuals exposed to such an environment may experience symptoms such as headaches, ringing in the ears, dizziness, drowsiness, unconsciousness, nausea, vomiting, and depression of all senses.
Environmental Effects of Hydrogen
- Hydrogen makes up 0.15% of the earth’s crust and is the most abundant element in water. The atmosphere contains 0.5 ppm of hydrogen H2 and various proportions of water vapor. Hydrogen is also a significant component of biomass, accounting for 14% by weight.
- Any effect on plants or animals would be related to oxygen deficient environments. Except for frost produced in the presence of rapidly expanding gases, no adverse effects on plant life.
- There is currently no evidence that hydrogen has an effect on aquatic life.
Toxicity, Safety, and Precautions
- Highly flammable – Many reactions can result in a fire or explosion. Gas/air mixtures are highly flammable.
- The substance gets absorbed into the body through inhalation. Exposure to high concentrations of this gas can result in an oxygen-depleted environment
- Individuals exposed to such an environment with an oxygen-depleted condition may experience symptoms such as headaches, ringing in the ears, dizziness, drowsiness, unconsciousness, nausea, vomiting, and sensory depression.
- It is not carcinogenic, embryotoxic, teratogenic, or reproductively toxic.
- Overexposure to hydrogen may aggravate pre-existing respiratory conditions.
- When exposed to air, oxygen, halogens, and strong oxidants, it reacts violently, posing a fire and explosion risk. Metal catalysts, such as platinum and nickel, improve these reactions significantly.
Precautions
- Turn off the power; if this is not possible and there is no danger to the environment, let the fire burn out.
- Extinguish with water spray, powder, or carbon dioxide.
- In the event of an explosion, keep the cylinder cool by spraying it with water. Combat fire from a safe distance.
- Inhale the fresh air and relax.
- It is possible that artificial respiration will be required.
- Please seek medical attention.
Have a look on this interesting video about Hydrogen: Fuel of Future?
References
- “Hydrogen”. Van Nostrand’s Encyclopedia of Chemistry. Wylie-Interscience. 2005. pp. 797–799. ISBN 978-0-471-61525-5.
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. ISBN 978-0-8493-0464-4.
- Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. p. 240. ISBN 978-0123526519.
- https://www. britannica.com /science/ hydrogen/ Production-and-applications- of-hydrogen
- https://pubchem.ncbi.nlm.nih.gov/element/Hydrogen
- https://www.lenntech.com/periodic/elements/h.htm
- https://www.thoughtco.com/hydrogen-element-facts-606474
- https://byjus.com/chemistry/hydrogen/
- https://www.energy.gov/eere/fuelcells/safe-use-hydrogen